
How will you convert benzene to p-nitrotoluene?
Answer
527.1k+ views
Hint: First convert benzene to toluene. This can be one by Friedel Crafts alkylation. Then convert toluene to p-nitrotoluene by treating it with nitrating mixture.
Complete step by step answer:
Step 1: Convert benzene to toluene by Friedel Crafts alkylation as follows:
Benzene reacts with methyl chloride in presence of anhydrous aluminum chloride to give toluene. The reaction is,
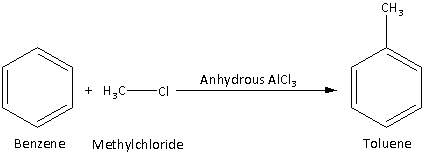
Step 2: Convert toluene to p-nitrotoluene by treating toluene with nitrating mixture as follows:
-Toluene reacts with the nitrating mixture to give a mixture of p-nitrotoluene and o-nitrotoluene. The nitrating mixture is the mixture of dilute sulfuric acid and dilute nitric acid. The reaction is,
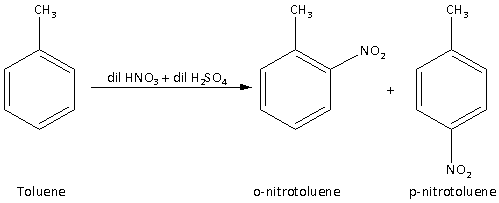
The mixture of p-nitrotoluene and o-nitrotoluene can be separated by fractional distillation.
Thus, benzene is converted p-nitrotoluene by converting benzene to toluene then toluene to a mixture of o-nitrotoluene and p-nitrotoluene and then separating the mixture by fractional distillation to get p-nitrotoluene.
Additional Information: The methyl group in toluene is an electron donating group. The methyl group releases electron density in the benzene ring. The extra electron density is concentrated to ortho and para positions. Thus, nitration of toluene becomes more easier than nitration of benzene. The nitration of toluene occurs 25 times faster than nitration of benzene.
Note: The methyl group in toluene is directly attached to the benzene ring. The methyl group increases the electron density at ortho and para positions on the benzene ring. Thus, toluene on nitration gives a mixture of o-nitrotoluene and p-nitrotoluene. On heating with a nitrating mixture, toluene gives dinitrotoluene and then finally trinitrotoluene. Trinitrotoluene (TNT) is explosive in nature.
Complete step by step answer:
Step 1: Convert benzene to toluene by Friedel Crafts alkylation as follows:
Benzene reacts with methyl chloride in presence of anhydrous aluminum chloride to give toluene. The reaction is,

Step 2: Convert toluene to p-nitrotoluene by treating toluene with nitrating mixture as follows:
-Toluene reacts with the nitrating mixture to give a mixture of p-nitrotoluene and o-nitrotoluene. The nitrating mixture is the mixture of dilute sulfuric acid and dilute nitric acid. The reaction is,

The mixture of p-nitrotoluene and o-nitrotoluene can be separated by fractional distillation.
Thus, benzene is converted p-nitrotoluene by converting benzene to toluene then toluene to a mixture of o-nitrotoluene and p-nitrotoluene and then separating the mixture by fractional distillation to get p-nitrotoluene.
Additional Information: The methyl group in toluene is an electron donating group. The methyl group releases electron density in the benzene ring. The extra electron density is concentrated to ortho and para positions. Thus, nitration of toluene becomes more easier than nitration of benzene. The nitration of toluene occurs 25 times faster than nitration of benzene.
Note: The methyl group in toluene is directly attached to the benzene ring. The methyl group increases the electron density at ortho and para positions on the benzene ring. Thus, toluene on nitration gives a mixture of o-nitrotoluene and p-nitrotoluene. On heating with a nitrating mixture, toluene gives dinitrotoluene and then finally trinitrotoluene. Trinitrotoluene (TNT) is explosive in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




