
Convert calcium salt of acid to aldehyde and ketone.
Answer
563.4k+ views
Hint:Dry distillation is the process where the material is heated and converted into vapor forms. Pyrolysis or thermolysis is carried out in dry distillation where a high temperature is used to carry out the reaction. Dry distillation is used to convert calcium salt dicarboxylic acid into aldehyde and ketone. Depending upon the salt used either symmetric or asymmetric product is formed.
Complete solution:
The calcium salt on dry distillation gives the aldehyde or ketone and calcium carbonate as products. The general mechanism of the reaction is as follows:
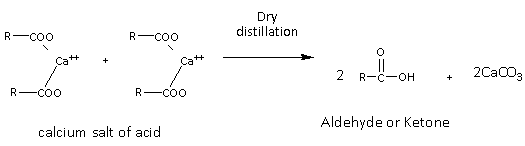
Here, the product of the reaction depends on the calcium salt used in the reaction. If 2 moles of the same salt are used then the product formed is symmetric while if two different calcium salts are used then the product formed is asymmetric.
Here, R represents any alkyl group or H atom. If R is H then the calcium salt of the acid is calcium format and it gives formaldehyde. The reaction is as follows:
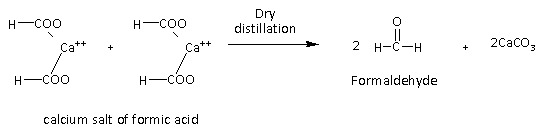
Here, we can see that if the salt is formic acid then it gives formaldehyde as shown in the above reaction.
If R is a methyl group then the product obtained is the ketone and the reaction is as follows:
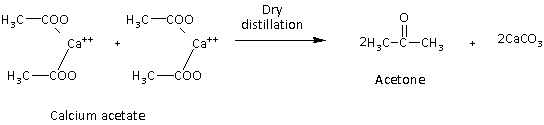
Here, we can see that if two moles of calcium salt other than the calcium format are used then it forms a ketone.
If two different calcium salts are used then the product obtained is asymmetric. The reaction is as follows:
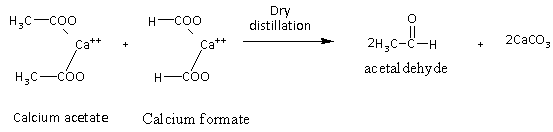
Here, we can see that if we obtain the aldehyde other than the formaldehyde use two different calcium salt molecules in such a way that one is always calcium salt of formic acid and the other molecule is of any other calcium salt molecule.
In this way, we can convert the calcium salt of acid into the aldehyde and ketone.
Note: Dry distillation is also referred to as pyrolysis reaction. To obtain the symmetric aldehyde and ketone use the two molecules of the same calcium salt. If we want to obtain the asymmetric aldehyde or ketone using two molecules of different calcium salt of acids.
Complete solution:
The calcium salt on dry distillation gives the aldehyde or ketone and calcium carbonate as products. The general mechanism of the reaction is as follows:

Here, the product of the reaction depends on the calcium salt used in the reaction. If 2 moles of the same salt are used then the product formed is symmetric while if two different calcium salts are used then the product formed is asymmetric.
Here, R represents any alkyl group or H atom. If R is H then the calcium salt of the acid is calcium format and it gives formaldehyde. The reaction is as follows:

Here, we can see that if the salt is formic acid then it gives formaldehyde as shown in the above reaction.
If R is a methyl group then the product obtained is the ketone and the reaction is as follows:

Here, we can see that if two moles of calcium salt other than the calcium format are used then it forms a ketone.
If two different calcium salts are used then the product obtained is asymmetric. The reaction is as follows:

Here, we can see that if we obtain the aldehyde other than the formaldehyde use two different calcium salt molecules in such a way that one is always calcium salt of formic acid and the other molecule is of any other calcium salt molecule.
In this way, we can convert the calcium salt of acid into the aldehyde and ketone.
Note: Dry distillation is also referred to as pyrolysis reaction. To obtain the symmetric aldehyde and ketone use the two molecules of the same calcium salt. If we want to obtain the asymmetric aldehyde or ketone using two molecules of different calcium salt of acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




