
How would you convert cyclohexanol to chlorocyclohexane?
Answer
555.3k+ views
Hint: Cyclohexanol is a molecule having a chemical formula, ${{C}_{6}}{{H}_{12}}O$ . In the molecule of cyclohexane, one hydrogen atom is replaced by one hydroxyl group, which results in the formation of cyclohexanol. It is a colorless solid, which has a camphor like odour.
Complete step-by-step answer:When cyclohexanol is reacted with thionyl chloride, it results in the formation of chlorocyclohexane. The byproducts formed during this reaction are sulphur dioxide and hydrogen chloride. They are present in gaseous form and can be removed easily during the reaction. This is why this method is a preferred method when an alcohol is converted into alkyl chloride.
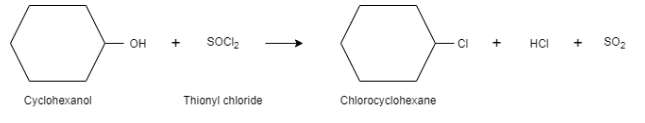
In this reaction, the hydroxyl group acts as a leaving group and chloride ion is the nucleophile. In this reaction, a bond is broken between carbon atom and oxygen atom and the new bond is formed between carbon atom and chlorine atom. It is a substitution reaction that follows the $S{{N}^{2}}$ mechanism. In the first step, the oxygen atom of cyclohexanol attacks on the sulphur of thionyl chloride, which results in the displacement of chloride ions. Here, the alcohol acts as a good leaving group.
In the second step, the chloride ion attacks the carbon, which results in the breakage of the carbon – oxygen bond. This shows $S{{N}^{2}}$ mechanism.
Note:The above reaction is a substitution reaction in which one functional group of a compound is replaced by another functional group.
The $S{{N}^{2}}$ reaction is the elimination of the leaving group and a nucleophile is added simultaneously in the reaction. $S{{N}^{2}}$ reaction only takes place when a nucleophile has the excess to the central carbon atom.
Complete step-by-step answer:When cyclohexanol is reacted with thionyl chloride, it results in the formation of chlorocyclohexane. The byproducts formed during this reaction are sulphur dioxide and hydrogen chloride. They are present in gaseous form and can be removed easily during the reaction. This is why this method is a preferred method when an alcohol is converted into alkyl chloride.

In this reaction, the hydroxyl group acts as a leaving group and chloride ion is the nucleophile. In this reaction, a bond is broken between carbon atom and oxygen atom and the new bond is formed between carbon atom and chlorine atom. It is a substitution reaction that follows the $S{{N}^{2}}$ mechanism. In the first step, the oxygen atom of cyclohexanol attacks on the sulphur of thionyl chloride, which results in the displacement of chloride ions. Here, the alcohol acts as a good leaving group.
In the second step, the chloride ion attacks the carbon, which results in the breakage of the carbon – oxygen bond. This shows $S{{N}^{2}}$ mechanism.
Note:The above reaction is a substitution reaction in which one functional group of a compound is replaced by another functional group.
The $S{{N}^{2}}$ reaction is the elimination of the leaving group and a nucleophile is added simultaneously in the reaction. $S{{N}^{2}}$ reaction only takes place when a nucleophile has the excess to the central carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




