
How will you convert ethyl chloride into
(i) Ethane
(ii) n-butane
Answer
577.5k+ views
Hint: A lot of applications are there for alkyl halides. Generally alkyl halides react with alcoholic KOH and produce alkenes and by using alkyl halides we can prepare higher alkanes through Wurtz reaction using sodium metal.
Complete step by step solution:
- In the question it asked to prepare ethane and n-butane from ethyl chloride.
(i) Ethane from ethyl chloride.
- Preparation of ethane from ethyl chloride is a two-step process.
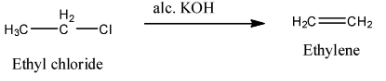
- The reaction of ethyl chloride with alcoholic KOH produces an alkene in the first step and it is as follows. It is an example for elimination reaction.
(a).
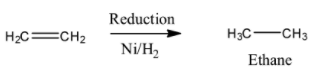
- The formed ethylene undergoes reduction reaction in presence of Nickel catalyst gives ethane as the product in the second step.
(b).
(ii) n-butane from ethyl chloride.
- Ethyl chloride reacts with sodium metal and forms a higher alkane and it is as follows.
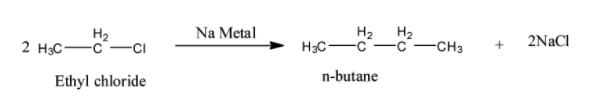
- In the above reaction two moles of Ethyl chloride react with sodium metal and form one moles of n-butane and two moles of sodium chloride as the products.
- The above reaction is called Wurtz reaction.
- The reaction occurs in the presence of sodium metal.
- Wurtz reaction is one of the best methods to prepare higher alkanes from alkyl halides.
Note: Alkyl halides have a lot of applications in organic chemistry. By using alkyl halides we can prepare alkanes that have the same number of carbon atoms and we can prepare higher alkanes using sodium metal through Wurtz reaction.
Complete step by step solution:
- In the question it asked to prepare ethane and n-butane from ethyl chloride.
(i) Ethane from ethyl chloride.
- Preparation of ethane from ethyl chloride is a two-step process.
- The reaction of ethyl chloride with alcoholic KOH produces an alkene in the first step and it is as follows. It is an example for elimination reaction.
(a).

- The formed ethylene undergoes reduction reaction in presence of Nickel catalyst gives ethane as the product in the second step.
(b).

(ii) n-butane from ethyl chloride.
- Ethyl chloride reacts with sodium metal and forms a higher alkane and it is as follows.

- In the above reaction two moles of Ethyl chloride react with sodium metal and form one moles of n-butane and two moles of sodium chloride as the products.
- The above reaction is called Wurtz reaction.
- The reaction occurs in the presence of sodium metal.
- Wurtz reaction is one of the best methods to prepare higher alkanes from alkyl halides.
Note: Alkyl halides have a lot of applications in organic chemistry. By using alkyl halides we can prepare alkanes that have the same number of carbon atoms and we can prepare higher alkanes using sodium metal through Wurtz reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




