
How will you convert:
(i) Propene to Propan-2-ol?
(ii) Phenol to 2,4,6- trinitrophenol?
Answer
585.6k+ views
Hint: Recall the rules of addition reactions of unsymmetrical alkenes. 2,4,6- trinitrophenol is also known as Picric acid which is a carbon-nitro compound comprising phenol having three substituents at 2,4 and 6 positions.
Complete step by step answer:
-(i) To begin the conversions, we must recall the concept of Markovnikov’s rule. Markovnikov’s rule tells us that in an asymmetrical alkene where to add the nucleophile and hydrogen through an addition reaction. The rule says that the nucleophile will add to the more substituted carbon, leaving the hydrogen to add on the less substituted carbon.
-Applying Markovnikov’s rule for the addition of sulphuric acid to Propene giving Propan-2-ol.
$C{{H}_{3}}CH=C{{H}_{2}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{boiling\text{ }{{H}_{2}}O}C{{H}_{3}}-C{{H}_{2}}OH-C{{H}_{3}}$
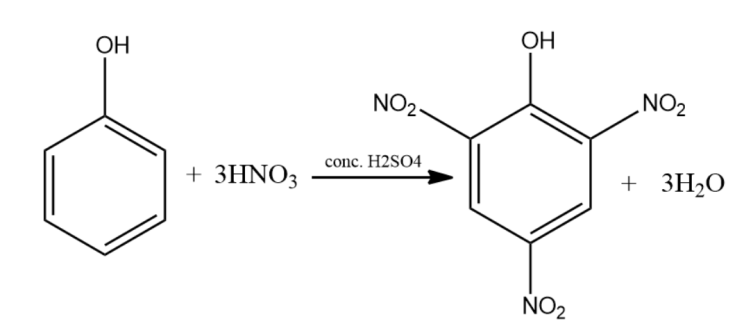
-(ii) Addition of concentrated Nitric acid to Phenol gets us 2,4,6-trinitrophenol or Picric acid. The electrophile nitronium ion substituted the hydrogen from all the ortho and para positions of the phenol.
Additional Information:
By remembering the phrase, ‘The rich get richer’, we can recall Markovnikov's reaction. In an alkene addition, the hydrogen will add to the carbon which is less substituted and so that the nucleophile can attack the carbon with more substituents.
While conducting a reaction for the production of picric acid, we must first perform sulphonation as it is extremely exothermic. The direct addition of even dilute nitric acid to phenol is very vigorous and dangerous. Picric acid is widely used for the production of explosives like TNT and Dunnite (Explosive-D).
Note: It is very important to learn the mechanism of Markovnikov’s rule because the addition of nucleophiles depends on the formation of most stable intermediates. There are some reactions also, where no addition of hydrogen takes place.
Complete step by step answer:
-(i) To begin the conversions, we must recall the concept of Markovnikov’s rule. Markovnikov’s rule tells us that in an asymmetrical alkene where to add the nucleophile and hydrogen through an addition reaction. The rule says that the nucleophile will add to the more substituted carbon, leaving the hydrogen to add on the less substituted carbon.
-Applying Markovnikov’s rule for the addition of sulphuric acid to Propene giving Propan-2-ol.
$C{{H}_{3}}CH=C{{H}_{2}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{boiling\text{ }{{H}_{2}}O}C{{H}_{3}}-C{{H}_{2}}OH-C{{H}_{3}}$
-(ii) Addition of concentrated Nitric acid to Phenol gets us 2,4,6-trinitrophenol or Picric acid. The electrophile nitronium ion substituted the hydrogen from all the ortho and para positions of the phenol.

Additional Information:
By remembering the phrase, ‘The rich get richer’, we can recall Markovnikov's reaction. In an alkene addition, the hydrogen will add to the carbon which is less substituted and so that the nucleophile can attack the carbon with more substituents.
While conducting a reaction for the production of picric acid, we must first perform sulphonation as it is extremely exothermic. The direct addition of even dilute nitric acid to phenol is very vigorous and dangerous. Picric acid is widely used for the production of explosives like TNT and Dunnite (Explosive-D).
Note: It is very important to learn the mechanism of Markovnikov’s rule because the addition of nucleophiles depends on the formation of most stable intermediates. There are some reactions also, where no addition of hydrogen takes place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




