
How will you convert the following?
Aniline to fluorobenzene.
Answer
561k+ views
Hint: Aniline can be converted into fluorobenzene by first converting aniline to benzene diazonium chloride and then the benzene diazonium chloride to fluorobenzene. Overall reaction will be the replacement of amine group in benzene ring by a fluorine atom.
Complete step by step answer:
Aniline is an aromatic compound in which an $ - N{H_2}$ group is attached to a benzene ring. In fluorobenzene, instead of this $ - N{H_2}$ group, a fluorine atom is present. The conversion of aniline to fluorobenzene involves two steps. Let us discuss each step in detail.
i.Conversion of aniline to benzene diazonium chloride.
When aniline is mixed with dilute hydrochloric acid (HCl) and an aqueous solution of sodium nitrate $(NaN{O_2})$ at a temperature $273 - 278K$ , benzene diazonium chloride is formed. This reaction is called diazotization reaction. Diazotization reactions are usually referred to as the conversion of primary aromatic amine to corresponding diazonium salt. The reaction is given below.
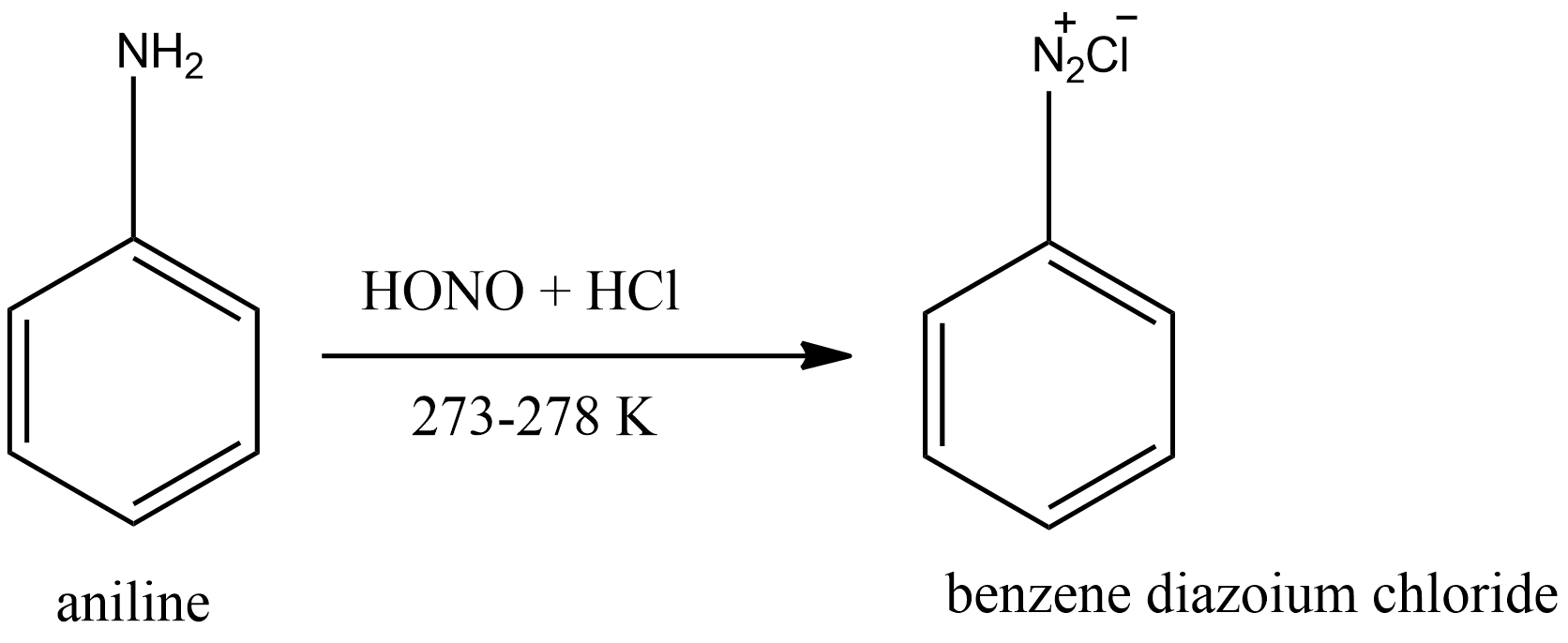
First sodium nitrate reacts with HCl to form $HN{O_2}$ . It then produces an electrophile and attacks the aniline.
ii.Conversion of benzene diazonium chloride to fluorobenzene.
The benzene diazonium chloride formed is then treated with fluoroboric acid $(HB{F_4})$ to yield fluorobenzene. This reaction also requires heat. The reaction is shown below.
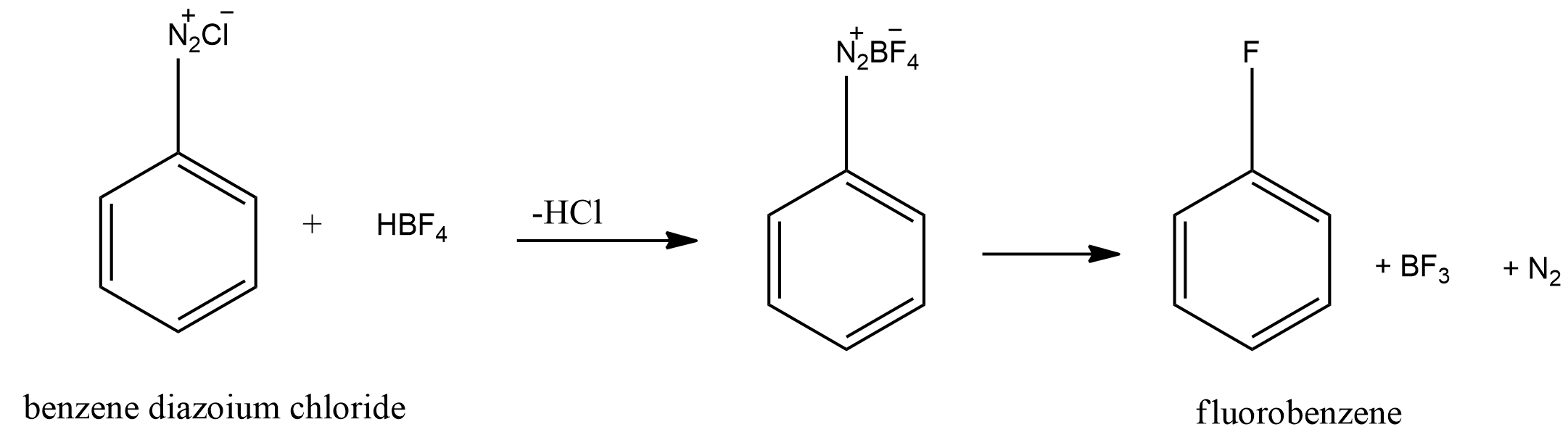
First the chloride ion in benzene diazonium chloride is replaced with $B{F_4}^ - $ . The resulting compound eliminates nitrogen gas and boron trifluoride to give fluorobenzene.
Note:
This is a common method of preparation of fluorobenzene. Fluorobenzene is a colourless liquid and its solubility in water is low. Fluorobenzene behaves differently compared to other halobenzenes because of pi-donor properties of fluorine.
Complete step by step answer:
Aniline is an aromatic compound in which an $ - N{H_2}$ group is attached to a benzene ring. In fluorobenzene, instead of this $ - N{H_2}$ group, a fluorine atom is present. The conversion of aniline to fluorobenzene involves two steps. Let us discuss each step in detail.
i.Conversion of aniline to benzene diazonium chloride.
When aniline is mixed with dilute hydrochloric acid (HCl) and an aqueous solution of sodium nitrate $(NaN{O_2})$ at a temperature $273 - 278K$ , benzene diazonium chloride is formed. This reaction is called diazotization reaction. Diazotization reactions are usually referred to as the conversion of primary aromatic amine to corresponding diazonium salt. The reaction is given below.

First sodium nitrate reacts with HCl to form $HN{O_2}$ . It then produces an electrophile and attacks the aniline.
ii.Conversion of benzene diazonium chloride to fluorobenzene.
The benzene diazonium chloride formed is then treated with fluoroboric acid $(HB{F_4})$ to yield fluorobenzene. This reaction also requires heat. The reaction is shown below.

First the chloride ion in benzene diazonium chloride is replaced with $B{F_4}^ - $ . The resulting compound eliminates nitrogen gas and boron trifluoride to give fluorobenzene.
Note:
This is a common method of preparation of fluorobenzene. Fluorobenzene is a colourless liquid and its solubility in water is low. Fluorobenzene behaves differently compared to other halobenzenes because of pi-donor properties of fluorine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




