
Convert the following:
(i) Ethanol to But$ - 2 - $yne
(ii) But $ - 1 - $ene to n-butyl iodide
(iii) Ethanol to propane nitrile
Answer
526.7k+ views
Hint : We can convert the given compounds by following a set of reactions. In the conversion of compounds we should be aware of the number of carbon products and reactants which help in the writing the conversion equation.
Complete step by step solution:
(i) Ethanol to But$ - 2 - $yne – To convert ethanol to but$ - 2 - $yne by following order of reactions. First of halogenation of ethanol will be performed with $P/B{r_2}$,then dehydrohalogenation reaction. After that electrophilic substitution will be done. Then at last nucleophilic substitution will happen which will produce but$ - 2 - $yne. So the reactions are given below in the order;
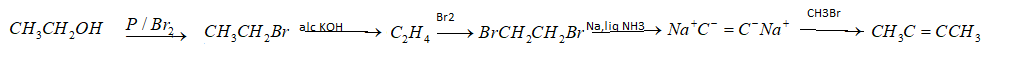
(ii) But $ - 1 - $ene to n-butyl iodide – When but $ - 1 - $ene will react with hydrogen bromide in presence peroxide it will produce n- butyl bromide. After getting n- butyl bromide with the help of Finkelstein reaction we can get n butyl iodide. This reaction is carried out with $NaI$ and acetone. Hence the reaction is as given below;
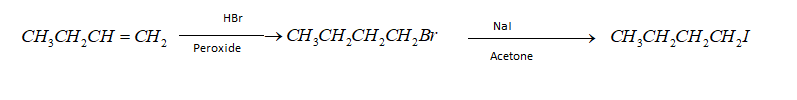
(iii) Ethanol to propane nitrile – To convert Ethanol to propane nitrile first of all ethanol is converted to bromoethane in the presence of catalyst red $P/B{r_2}$ then bromomethane is converted to propane nitrile with the help of catalyst $KCN$/ aqueous ethanol as catalyst. Hence the following reactions will take place in the conversion;
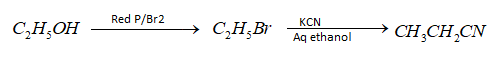
Note: Hence have obtained the desired product given in the problem. To convert organic compounds we should revise the equation which can increase or decrease the number of carbon in reaction as this trick is very helpful in the synthesis of organic compounds.
Complete step by step solution:
(i) Ethanol to But$ - 2 - $yne – To convert ethanol to but$ - 2 - $yne by following order of reactions. First of halogenation of ethanol will be performed with $P/B{r_2}$,then dehydrohalogenation reaction. After that electrophilic substitution will be done. Then at last nucleophilic substitution will happen which will produce but$ - 2 - $yne. So the reactions are given below in the order;
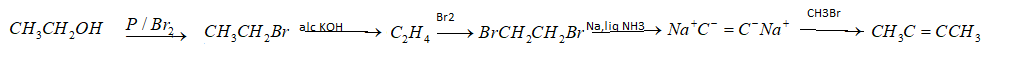
(ii) But $ - 1 - $ene to n-butyl iodide – When but $ - 1 - $ene will react with hydrogen bromide in presence peroxide it will produce n- butyl bromide. After getting n- butyl bromide with the help of Finkelstein reaction we can get n butyl iodide. This reaction is carried out with $NaI$ and acetone. Hence the reaction is as given below;
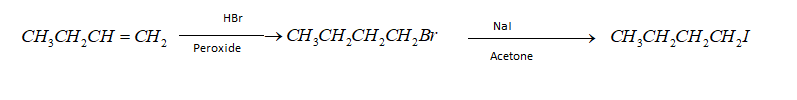
(iii) Ethanol to propane nitrile – To convert Ethanol to propane nitrile first of all ethanol is converted to bromoethane in the presence of catalyst red $P/B{r_2}$ then bromomethane is converted to propane nitrile with the help of catalyst $KCN$/ aqueous ethanol as catalyst. Hence the following reactions will take place in the conversion;
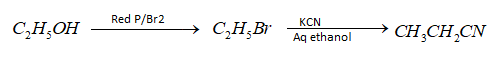
Note: Hence have obtained the desired product given in the problem. To convert organic compounds we should revise the equation which can increase or decrease the number of carbon in reaction as this trick is very helpful in the synthesis of organic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




