
How will you convert the following in not more than two steps:
(i) Benzoic acid to Benzaldehyde
(ii) Acetophenone to benzoic acid
(iii) Ethanoic acid to 2- Hydroxyethanoic acid
Answer
595.2k+ views
Hint: We know that organic compounds can be synthesized with the help of organic reactions. But here it is mentioned in the question that the conversion should not be more than two steps. There are many reactions in chemistry that would give the desired compound.
Complete step by step answer:
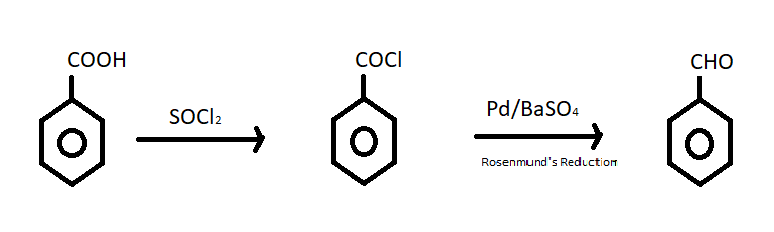
(i) Benzoic acid to Benzaldehyde – We have studied that when benzoic acid is treated with thionyl chloride or \[SOC{l_2}\] it is converted into benzoyl chloride. Then in second step acid chloride (benzoyl chloride) reacts with $Pd/BaS{O_4}$ and produces benzaldehyde. This reaction is known as Rosenmund’s reduction reaction. The reaction of conversion is given below;
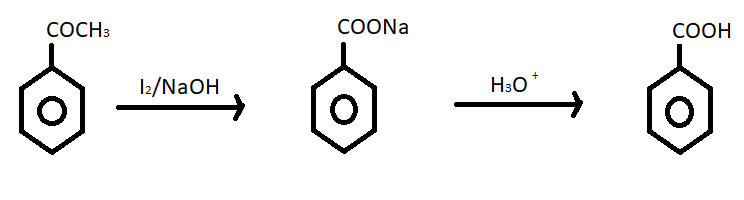
(ii) Acetophenone to benzoic acid – For converting acetophenone to benzoic acid in two step ; first we treat acetophenone with ${I_2}/NaOH$ it produces sodium benzoate or ${C_6}{H_5}COONa$. In the second step sodium benzoate reacts with ${H_3}{O^ + }$ and it gives benzoic acid. The reaction of conversion of acetophenone to benzoic acid is given below;
(iii) Ethanoic acid to 2- Hydroxyethanoic acid - Ethanoic acid or acetic acid when reacts with chlorine in the presence of red phosphorus it forms mono chloro acetic acid . This reaction is also known as HVZ reaction or Hell Volhard Zelinsky reaction. Then $Cl - C{H_2}COOH$ further reacts with aqueous potassium hydroxide and gives 2- Hydroxyethanoic acid. The reaction is given below;
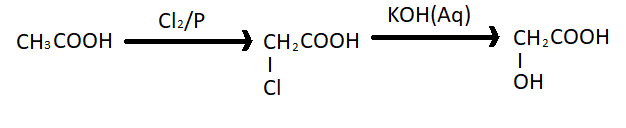
Note:
1-We have approached the problem with the help of some important and famous organic reactions of chemistry such as for the conversion of acetophenone to sodium benzoate we have used iodoform reaction.
2-We should remember these name reactions as they play an important role in the conversion reactions of compounds.
Complete step by step answer:
(i) Benzoic acid to Benzaldehyde – We have studied that when benzoic acid is treated with thionyl chloride or \[SOC{l_2}\] it is converted into benzoyl chloride. Then in second step acid chloride (benzoyl chloride) reacts with $Pd/BaS{O_4}$ and produces benzaldehyde. This reaction is known as Rosenmund’s reduction reaction. The reaction of conversion is given below;

(ii) Acetophenone to benzoic acid – For converting acetophenone to benzoic acid in two step ; first we treat acetophenone with ${I_2}/NaOH$ it produces sodium benzoate or ${C_6}{H_5}COONa$. In the second step sodium benzoate reacts with ${H_3}{O^ + }$ and it gives benzoic acid. The reaction of conversion of acetophenone to benzoic acid is given below;

(iii) Ethanoic acid to 2- Hydroxyethanoic acid - Ethanoic acid or acetic acid when reacts with chlorine in the presence of red phosphorus it forms mono chloro acetic acid . This reaction is also known as HVZ reaction or Hell Volhard Zelinsky reaction. Then $Cl - C{H_2}COOH$ further reacts with aqueous potassium hydroxide and gives 2- Hydroxyethanoic acid. The reaction is given below;
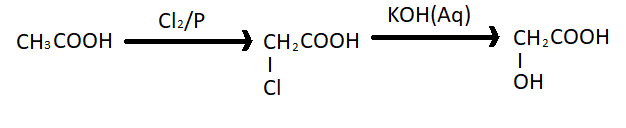
Note:
1-We have approached the problem with the help of some important and famous organic reactions of chemistry such as for the conversion of acetophenone to sodium benzoate we have used iodoform reaction.
2-We should remember these name reactions as they play an important role in the conversion reactions of compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




