
What would be correct about bonding in chloral hydrate \[CC{{l}_{3}}CH{{(OH)}_{2}}\] ?
A.Only intramolecular H-bonding
B.Only intermolecular H-bonding
C.Both inter and intramolecular H-bonding
D.No hydrogen bonding
Answer
502.2k+ views
Hint: This compound that is given in the question is an exception in chemistry and hydrogen bonding, as chlorine does not generally show hydrogen bonding but in this molecule it shows hydrogen bonding.
Complete answer:
The correct answer to this question is option A, only intramolecular H-bonding occurs in chloral hydrate, that is \[CC{{l}_{3}}CH{{(OH)}_{2}}\] .
First, we have to understand the difference between intermolecular hydrogen bonding and intramolecular hydrogen bonding.
Intermolecular hydrogen bonding occurs between two same or different kinds of molecule, and intra molecular hydrogen bonding occurs between the atoms of the same molecule.
Thus, let us take a look at the structure of chloral hydrate, that is \[CC{{l}_{3}}CH{{(OH)}_{2}}\] .
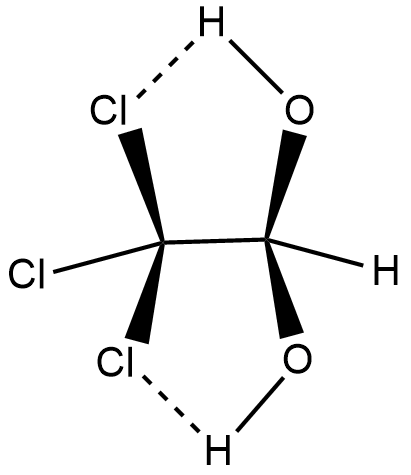
As shown in the above figure, the molecule of chloral hydrate comprises carbon, chlorine, hydrogen and oxygen. Carbon is not shown in the figure but it is there between the bonds of chlorines and oxygens.
The dotted line in the above figure shows hydrogen bonding between chlorine and hydrogen. Therefore, the compound chloral hydrate contains an intra molecular hydrogen bond.
Thus, option D, which says the compound does not contain any type of hydrogen bond, gets automatically discarded.
But, the compound does not contain an intermolecular hydrogen bond, thus the answer is option A, chloral hydrate only contains intra molecular hydrogen bond.
Note:
Chlorine does not show hydrogen bonding due to its electron density which comes from the size of its atom. Despite chlorine being highly electronegative, it does not form hydrogen bonding, neither intra nor inter. This example is an exception to that statement, a reason is believed that as the given compound is a geminal diol. Geminal diols are generally unstable compounds, so to make it stable, chlorine might have formed an intramolecular hydrogen bond.
Complete answer:
The correct answer to this question is option A, only intramolecular H-bonding occurs in chloral hydrate, that is \[CC{{l}_{3}}CH{{(OH)}_{2}}\] .
First, we have to understand the difference between intermolecular hydrogen bonding and intramolecular hydrogen bonding.
Intermolecular hydrogen bonding occurs between two same or different kinds of molecule, and intra molecular hydrogen bonding occurs between the atoms of the same molecule.
Thus, let us take a look at the structure of chloral hydrate, that is \[CC{{l}_{3}}CH{{(OH)}_{2}}\] .

As shown in the above figure, the molecule of chloral hydrate comprises carbon, chlorine, hydrogen and oxygen. Carbon is not shown in the figure but it is there between the bonds of chlorines and oxygens.
The dotted line in the above figure shows hydrogen bonding between chlorine and hydrogen. Therefore, the compound chloral hydrate contains an intra molecular hydrogen bond.
Thus, option D, which says the compound does not contain any type of hydrogen bond, gets automatically discarded.
But, the compound does not contain an intermolecular hydrogen bond, thus the answer is option A, chloral hydrate only contains intra molecular hydrogen bond.
Note:
Chlorine does not show hydrogen bonding due to its electron density which comes from the size of its atom. Despite chlorine being highly electronegative, it does not form hydrogen bonding, neither intra nor inter. This example is an exception to that statement, a reason is believed that as the given compound is a geminal diol. Geminal diols are generally unstable compounds, so to make it stable, chlorine might have formed an intramolecular hydrogen bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




