
Correct energy profile for amine inversion and hybridization of nitrogen in transition state is:
A.
B.
C.
D.





Answer
568.5k+ views
Hint:
-When an amine group undergoes the process of inversion, the groups attached to the nitrogen atom turn upside down, in other words their spatial arrangement inverts.
-The inverted structure of a molecule is an enantiomer as it is the mirror image of that molecule and the energy of that molecule doesn’t change, as no energy is released or absorbed in the process.
Complete step by step answer:
We all know that the outermost orbital of the nitrogen contains five electrons, so it can form three bonds with any group. So, the amine group has a hybridisation of $s{{p}^{3}}$, since one $s$ orbital and three $p$ orbitals are involved in the bonding.
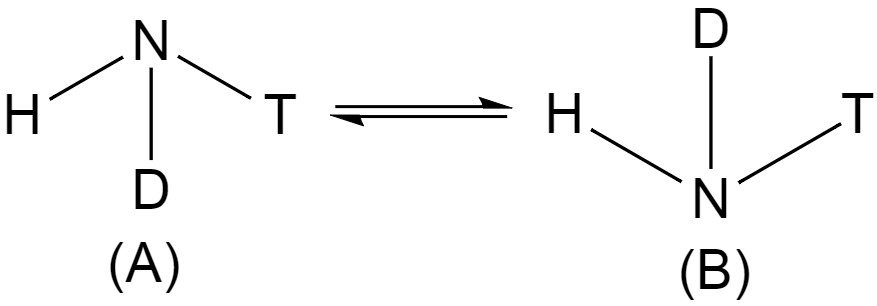
During the inversion of an amine, the \[~C3\] axis of the amine is presented as horizontal, and the dots in pair signifies the lone pair of the nitrogen atom collinear with that axis. In chemistry, nitrogen inversion is a fluxional process in the nitrogen and amines, where the whole molecule "turns inside out".
During the state of transition the amine is $s{{p}^{2}}$ hybridised, as the lone pair enters the nucleus, which leaves the orbital of the amine vacant.
And in the process of inversion, the molecule becomes a mirror image of each other or it becomes enantiomers, so the process would not release or absorb any energy, in other words, the energy of the molecule will remain the same.
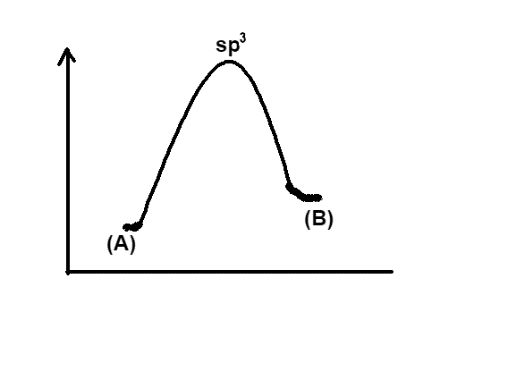
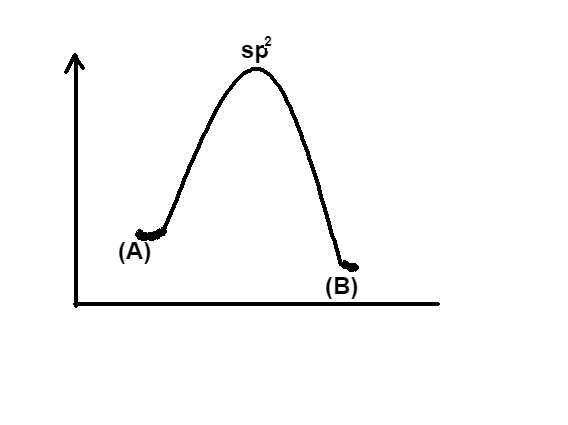
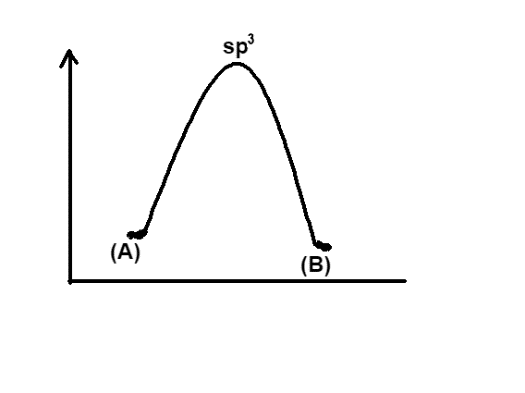
Now if we look at the graph given in option A, it is clear that the energy of (A) is higher than that of (B), in other words this graph shows that energy is released in the process. So this option is incorrect. In a similar manner, look at the graph given in option B, the energy of (B) is higher than that of (A) so this option would also be incorrect. The option C, similar to the option A, says that the energy of the reactant is more, hence it is incorrect.
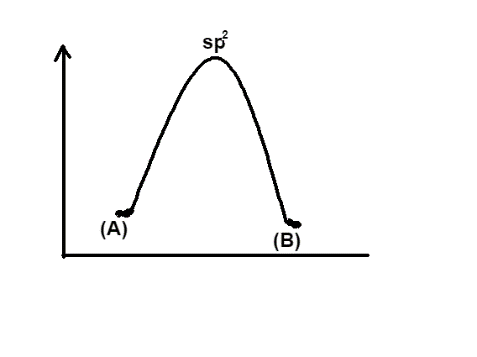
Finally, the option D represents the same energy levels of (A) and (B) and has hybridisation $s{{p}^{2}}$, so it will be the appropriate graph.
Hence the correct answer is option, D.
Note: The hybridisation of amine while undergoing the process of inversion, that is, in the transition state of amine, the hybridisation of nitrogen changes from $s{{p}^{3}}$ to $s{{p}^{2}}$ because the lone pairs of the nitrogen leaves the atom and a vacant space is created.
-But after the inversion is done, the energy of the molecule is unchanged, this is why the graph showing the same energy of (A) and (B) in the given question was correct.
-When an amine group undergoes the process of inversion, the groups attached to the nitrogen atom turn upside down, in other words their spatial arrangement inverts.
-The inverted structure of a molecule is an enantiomer as it is the mirror image of that molecule and the energy of that molecule doesn’t change, as no energy is released or absorbed in the process.
Complete step by step answer:
We all know that the outermost orbital of the nitrogen contains five electrons, so it can form three bonds with any group. So, the amine group has a hybridisation of $s{{p}^{3}}$, since one $s$ orbital and three $p$ orbitals are involved in the bonding.
During the inversion of an amine, the \[~C3\] axis of the amine is presented as horizontal, and the dots in pair signifies the lone pair of the nitrogen atom collinear with that axis. In chemistry, nitrogen inversion is a fluxional process in the nitrogen and amines, where the whole molecule "turns inside out".
During the state of transition the amine is $s{{p}^{2}}$ hybridised, as the lone pair enters the nucleus, which leaves the orbital of the amine vacant.
And in the process of inversion, the molecule becomes a mirror image of each other or it becomes enantiomers, so the process would not release or absorb any energy, in other words, the energy of the molecule will remain the same.
Now if we look at the graph given in option A, it is clear that the energy of (A) is higher than that of (B), in other words this graph shows that energy is released in the process. So this option is incorrect. In a similar manner, look at the graph given in option B, the energy of (B) is higher than that of (A) so this option would also be incorrect. The option C, similar to the option A, says that the energy of the reactant is more, hence it is incorrect.
Finally, the option D represents the same energy levels of (A) and (B) and has hybridisation $s{{p}^{2}}$, so it will be the appropriate graph.
Hence the correct answer is option, D.
Note: The hybridisation of amine while undergoing the process of inversion, that is, in the transition state of amine, the hybridisation of nitrogen changes from $s{{p}^{3}}$ to $s{{p}^{2}}$ because the lone pairs of the nitrogen leaves the atom and a vacant space is created.
-But after the inversion is done, the energy of the molecule is unchanged, this is why the graph showing the same energy of (A) and (B) in the given question was correct.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




