
Correct order of stability of carbanion
A. IV>I>III>II
B. III>II>I>IV
C. II>III>I>IV
D. IV>I>II>IV

Answer
579.9k+ views
Hint: The organic species which has four pairs of electrons and a negative charge on one of its carbon centers is known as carbanion. Electron withdrawing inductive effect increases the stability of carbanion while electron donating inductive effect decreases the stability of carbanion.
Complete step by step answer:
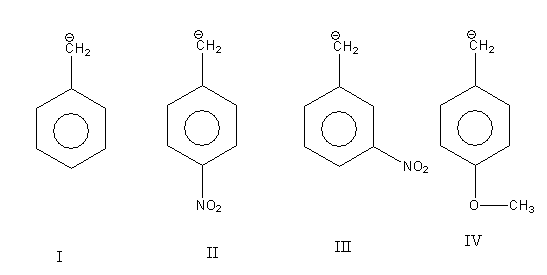
In all four compounds given to us carbanion is common only there is difference in substituent.
In structure I there is no substituent.
In structure II and III substituent$( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ is the same only there is a difference in the position they bonded to the aromatic ring. In structure II nitro group $( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ is para to $({\text{C}}{{\text{H}}_{\text{2}}}^{\text{ - }}{\text{)}}$ group while in structure III nitro group $( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ is meta to $({\text{C}}{{\text{H}}_{\text{2}}}^{\text{ - }}{\text{)}}$ group. The nitro group is an electron-withdrawing substituent.
In structure IV methoxy ${\text{( - OC}}{{\text{H}}_{\text{3}}}{\text{)}}$ substituent is bonded to an aromatic ring. It is para to $({\text{C}}{{\text{H}}_{\text{2}}}^{\text{ - }}{\text{)}}$ group. The methoxy group is an electron-donating substituent.
All possible resonance structures of carbanion are as follows:
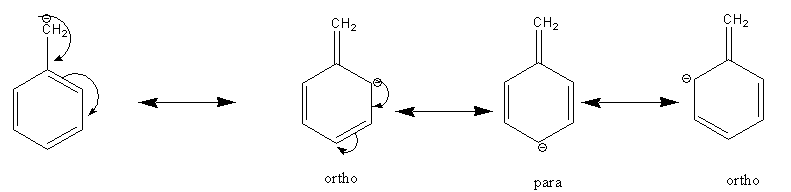
The resonance structures indicate that negative charge mobilized to the ortho and para position of the aromatic ring.
The electron-withdrawing group bonded to the ortho or para position of the aromatic ring increases the stability of carbanion. While the electron-donating group bonded to the ortho or para position of the aromatic ring decreases the stability of carbanion.
So, out of the given carbanion, carbanion IV is least stable as the electron-donating methoxy group ${\text{( - OC}}{{\text{H}}_{\text{3}}}{\text{)}}$present at para position decreases the stability of carbanion.
Carbanion I is more stable than carbanion IV as there is no electron-donating or electron-withdrawing substituent present in it.
Carbanion II is most stable as it contains an electron-withdrawing nitro group$( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ at the para position which increases the stability of carbanion.
Carbanion II also contains an electron-withdrawing nitro group$( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$but it is present at meta position so it is least stable than carbanion IV.
Thus, the decreasing order of stability of carbanion is as follows:
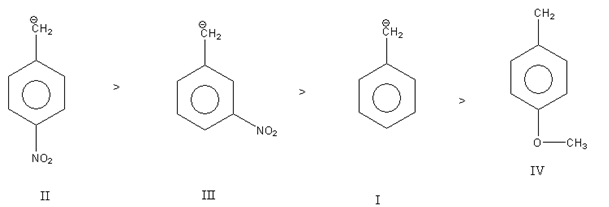
Thus, the correct option is C.
Note:
The stability of carbanion is dependent on the nature and position of the substituent. Electron withdrawing substituent increases the stability of carbanion and electron-donating substituent decreases the stability of carbanion.
Complete step by step answer:
In all four compounds given to us carbanion is common only there is difference in substituent.

In structure I there is no substituent.
In structure II and III substituent$( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ is the same only there is a difference in the position they bonded to the aromatic ring. In structure II nitro group $( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ is para to $({\text{C}}{{\text{H}}_{\text{2}}}^{\text{ - }}{\text{)}}$ group while in structure III nitro group $( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ is meta to $({\text{C}}{{\text{H}}_{\text{2}}}^{\text{ - }}{\text{)}}$ group. The nitro group is an electron-withdrawing substituent.
In structure IV methoxy ${\text{( - OC}}{{\text{H}}_{\text{3}}}{\text{)}}$ substituent is bonded to an aromatic ring. It is para to $({\text{C}}{{\text{H}}_{\text{2}}}^{\text{ - }}{\text{)}}$ group. The methoxy group is an electron-donating substituent.
All possible resonance structures of carbanion are as follows:

The resonance structures indicate that negative charge mobilized to the ortho and para position of the aromatic ring.
The electron-withdrawing group bonded to the ortho or para position of the aromatic ring increases the stability of carbanion. While the electron-donating group bonded to the ortho or para position of the aromatic ring decreases the stability of carbanion.
So, out of the given carbanion, carbanion IV is least stable as the electron-donating methoxy group ${\text{( - OC}}{{\text{H}}_{\text{3}}}{\text{)}}$present at para position decreases the stability of carbanion.
Carbanion I is more stable than carbanion IV as there is no electron-donating or electron-withdrawing substituent present in it.
Carbanion II is most stable as it contains an electron-withdrawing nitro group$( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$ at the para position which increases the stability of carbanion.
Carbanion II also contains an electron-withdrawing nitro group$( - {\text{N}}{{\text{O}}_{\text{2}}}{\text{)}}$but it is present at meta position so it is least stable than carbanion IV.
Thus, the decreasing order of stability of carbanion is as follows:

Thus, the correct option is C.
Note:
The stability of carbanion is dependent on the nature and position of the substituent. Electron withdrawing substituent increases the stability of carbanion and electron-donating substituent decreases the stability of carbanion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




