
Covalent compounds are soluble in:
A.Polar solvents
B.Non-polar solvents
C.Concentrated acids
D.All solvents
Answer
583.5k+ views
Hint: Covalent compounds are the ones having covalent bonds between the atoms in a molecule. A covalent bond is a chemical bond which involves the sharing of electrons between the atoms of a molecule. A covalent bond is sometimes also referred to as a molecular bond.
Complete step by step answer:
As stated above covalent compounds have covalent bonds which mean electrons are shared between the constituent atoms. Or in other words, we can say that electrons are neither completely donated nor gained by the atoms rather they are shared. Each of the two atoms involved in bond formation contributes one electron during the bond formation and to complete the octet of other atoms.
Since electrons are neither completely given nor taken but shared, there is nor polarity on either of the atoms which means these compounds are nonpolar or less polar.
Now as we all know, ‘Like dissolves like’, which means a polar compound will be dissolved in a polar solvent and a non-polar compound will get dissolved in a nonpolar solvent.
As discussed in the previous points covalent compounds are nonpolar nature, they will be soluble in a nonpolar solvent.
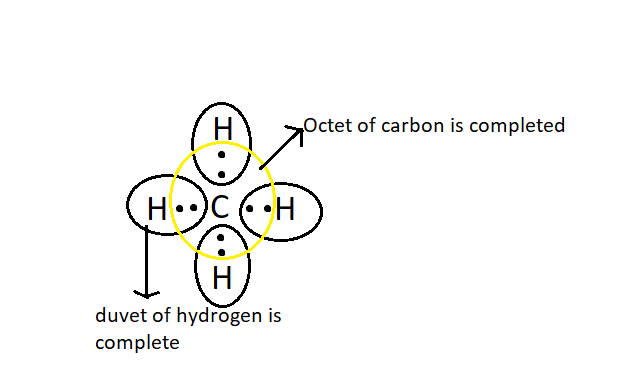
Let us look into some examples of covalent compound, ${\text{C}}{{\text{H}}_{\text{4}}}$ is a covalent compound. It has four covalent bonds between carbon and four hydrogen atoms. In each of C-H bond one electron is by hydrogen and one electron is shared by carbon, after getting one electron from Carbon hydrogen completes its duvet (it already had one electron and got one from carbon)
Similarly, carbon will get one electron from hydrogen, which means four electrons from four hydrogen atoms, thus since it already had 4 electrons and now got 4 more its octet is complete.
And hence option B is the correct answer.
Note:We have discussed covalent bond but there are two other types of bonds:
Ionic bond: in an ionic bond electron is either completely given or taken. For example, the bond between Na and Cl, one electron from Na is completely given to Cl, thus Na gaining positive charge and Cl attains negative charge. They are polar.
Coordination bond: In a coordination bond, only one of the two atoms gives both electrons.
Complete step by step answer:
As stated above covalent compounds have covalent bonds which mean electrons are shared between the constituent atoms. Or in other words, we can say that electrons are neither completely donated nor gained by the atoms rather they are shared. Each of the two atoms involved in bond formation contributes one electron during the bond formation and to complete the octet of other atoms.
Since electrons are neither completely given nor taken but shared, there is nor polarity on either of the atoms which means these compounds are nonpolar or less polar.
Now as we all know, ‘Like dissolves like’, which means a polar compound will be dissolved in a polar solvent and a non-polar compound will get dissolved in a nonpolar solvent.
As discussed in the previous points covalent compounds are nonpolar nature, they will be soluble in a nonpolar solvent.
Let us look into some examples of covalent compound, ${\text{C}}{{\text{H}}_{\text{4}}}$ is a covalent compound. It has four covalent bonds between carbon and four hydrogen atoms. In each of C-H bond one electron is by hydrogen and one electron is shared by carbon, after getting one electron from Carbon hydrogen completes its duvet (it already had one electron and got one from carbon)
Similarly, carbon will get one electron from hydrogen, which means four electrons from four hydrogen atoms, thus since it already had 4 electrons and now got 4 more its octet is complete.

And hence option B is the correct answer.
Note:We have discussed covalent bond but there are two other types of bonds:
Ionic bond: in an ionic bond electron is either completely given or taken. For example, the bond between Na and Cl, one electron from Na is completely given to Cl, thus Na gaining positive charge and Cl attains negative charge. They are polar.
Coordination bond: In a coordination bond, only one of the two atoms gives both electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




