
Why cyclooctatetraene is non-planar?
Answer
569.7k+ views
Hint: Draw the conformations of the cyclooctatetraene and try to analyze the lower energy conformation of the cyclooctatetraene. Also we can explain this with the internal angles of the structure that is why the molecule is not a planar molecule.
Complete Solution :
The structure the cyclooctatetraene is as shown below:
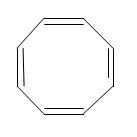
In the above shown figure the inter angles are greater than the 120 degrees. Even in the regular octagon the internal angles are 135 degrees but the angles of $s{{p}^{2}}$ hybridization is more stable at 120 degrees. And the bond length in the above structure is in between the length of the single and the double bond length. All these properties of the above structure show the non planarity of the molecule.
Also the lowest energy structure of the above cyclooctatetraene molecule is in the chair like form as shown below:
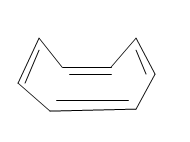
In the above lower energy conformation of the cyclooctatetraene all the carbons are not in the same plane or it is also said as the molecule is non-planar. Hence from the above structures and their properties showing the non planarity of the molecule we can conclude that the cyclooctatetraene is not a planar molecule.
Note: If the cyclooctatetraene is a planar molecule then it would be an antiaromatic compound because it is having $8\pi $ electrons. According to Huckel's rule there are $4n\pi $ electrons present in the planar molecule. Since the lower energy conformation is non planar the cyclooctatetraene molecule can also be considered as the non aromatic compound.
Complete Solution :
The structure the cyclooctatetraene is as shown below:

In the above shown figure the inter angles are greater than the 120 degrees. Even in the regular octagon the internal angles are 135 degrees but the angles of $s{{p}^{2}}$ hybridization is more stable at 120 degrees. And the bond length in the above structure is in between the length of the single and the double bond length. All these properties of the above structure show the non planarity of the molecule.
Also the lowest energy structure of the above cyclooctatetraene molecule is in the chair like form as shown below:

In the above lower energy conformation of the cyclooctatetraene all the carbons are not in the same plane or it is also said as the molecule is non-planar. Hence from the above structures and their properties showing the non planarity of the molecule we can conclude that the cyclooctatetraene is not a planar molecule.
Note: If the cyclooctatetraene is a planar molecule then it would be an antiaromatic compound because it is having $8\pi $ electrons. According to Huckel's rule there are $4n\pi $ electrons present in the planar molecule. Since the lower energy conformation is non planar the cyclooctatetraene molecule can also be considered as the non aromatic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




