
Define Fuel cell and write its two advantages.
Answer
576.9k+ views
Hint: In fuel cells, energy of combustion of fuel is converted to electrical energy. Water is only produced in reactions involved in fuel cells. Fuel cells have efficiency of 70%. In fuel cells, reaction of hydrogen with oxygen to produce water is used. In fuel cells, one of the reactants is fuel such as hydrogen or methanol.
Complete step by step solution:
-Producing electricity using thermal plants is a major source of pollution. Also, it is not a very efficient method to produce electricity.
-In thermal plants, from fossil fuels, chemical energy is obtained and is used in converting water to high pressure steam. This is used to run a turbine to produce electricity.
-Chemical energy is directly converted to electricity by galvanic cells and it is highly efficient.
-So galvanic cells are used to convert energy of combustion obtained from fuels like hydrogen, methane and methanol etc. directly into electricity such cells are called fuel cells.
-In this, reactants are continuously fed electrodes and products are continuously removed from the electrolyte compartment.
-In this cell, oxygen and hydrogen are continuously supplied through porous carbon electrodes.
-carbon electrodes are placed in electrolytes like sodium hydroxide or potassium hydroxide.
-Finely divided platinum metal is used as a catalyst which is incorporated in electrodes to increase the rate of reaction.
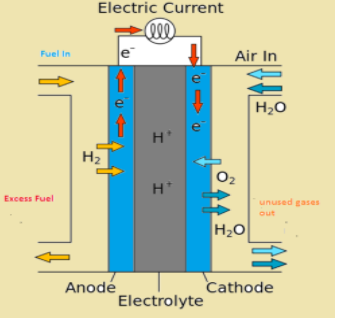
Reactions that take place at electrodes:
Cathode: ${{O}_{2}}+2{{H}_{2}}O+4{{e}^{-}}\to 4O{{H}^{-}}$
Anode: $2{{H}_{2}}+4O{{H}^{-}}\to 2{{H}_{2}}O$
Overall reaction: $2{{H}_{2}}+{{O}_{2}}\to 2{{H}_{2}}O$
Advantages: As reactants are continuously supplied, cell runs and there is no need to replace electrolytes.
The only product is water in fuel cells, it is non polluting. Fuel cells have efficiency of 70%. As compared to thermal plants.
Note: In fuel cells, energy of combustion of fuel is converted to electrical energy. The only product is water in fuel cells, it is non polluting. Producing electricity using thermal plants is a major source of pollution. Fuel cells have efficiency of 70%. As compared to thermal plants which have 40%. Fuel cell area used in automobiles. Chemical energy is directly converted to electrical energy in Fuel cells. Redox reactions take place in fuel cells.
Complete step by step solution:
-Producing electricity using thermal plants is a major source of pollution. Also, it is not a very efficient method to produce electricity.
-In thermal plants, from fossil fuels, chemical energy is obtained and is used in converting water to high pressure steam. This is used to run a turbine to produce electricity.
-Chemical energy is directly converted to electricity by galvanic cells and it is highly efficient.
-So galvanic cells are used to convert energy of combustion obtained from fuels like hydrogen, methane and methanol etc. directly into electricity such cells are called fuel cells.
-In this, reactants are continuously fed electrodes and products are continuously removed from the electrolyte compartment.
-In this cell, oxygen and hydrogen are continuously supplied through porous carbon electrodes.
-carbon electrodes are placed in electrolytes like sodium hydroxide or potassium hydroxide.
-Finely divided platinum metal is used as a catalyst which is incorporated in electrodes to increase the rate of reaction.

Reactions that take place at electrodes:
Cathode: ${{O}_{2}}+2{{H}_{2}}O+4{{e}^{-}}\to 4O{{H}^{-}}$
Anode: $2{{H}_{2}}+4O{{H}^{-}}\to 2{{H}_{2}}O$
Overall reaction: $2{{H}_{2}}+{{O}_{2}}\to 2{{H}_{2}}O$
Advantages: As reactants are continuously supplied, cell runs and there is no need to replace electrolytes.
The only product is water in fuel cells, it is non polluting. Fuel cells have efficiency of 70%. As compared to thermal plants.
Note: In fuel cells, energy of combustion of fuel is converted to electrical energy. The only product is water in fuel cells, it is non polluting. Producing electricity using thermal plants is a major source of pollution. Fuel cells have efficiency of 70%. As compared to thermal plants which have 40%. Fuel cell area used in automobiles. Chemical energy is directly converted to electrical energy in Fuel cells. Redox reactions take place in fuel cells.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




