
Describe the experiment conducted by Sir C.V Raman to demonstrate the Raman Effect. What is the use of this effect?
Answer
595.5k+ views
Hint: Firstly, we describe the Raman Effect as a change in the wavelength of light that occurs when molecules deflect a light beam. When a beam of l traverses a dust-free, transparent sample of a compound, a little fraction of the light emerges in directions aside from that of the incident (incoming) beam. It signifies as a tool for analyzing the composition of liquids, gases, and solids.
Complete step by step answer:
When a light beam is travelled through the substance, the light is reflected by the molecules of the essence. It means that the particles of the substance absorb photons of the light energy and subsequently emit light. In this process, the frequency of the absorbed and emitted light will exactly be the same. Thus the light coming out of the substance will have the same energy as that of the incident light; therefore, there is no energy loss, which is called Rayleigh scattering of light. But, Sir C. V. Raman observed a minimal volume (1 in 10 million parts) of the scattered light has a slightly different wavelength compared to the incident light wavelength. This change in the wavelength of the light beam is called a Raman Effect. It forms an essential part of spectroscopy. This happens due to some absorption of energy by molecules.
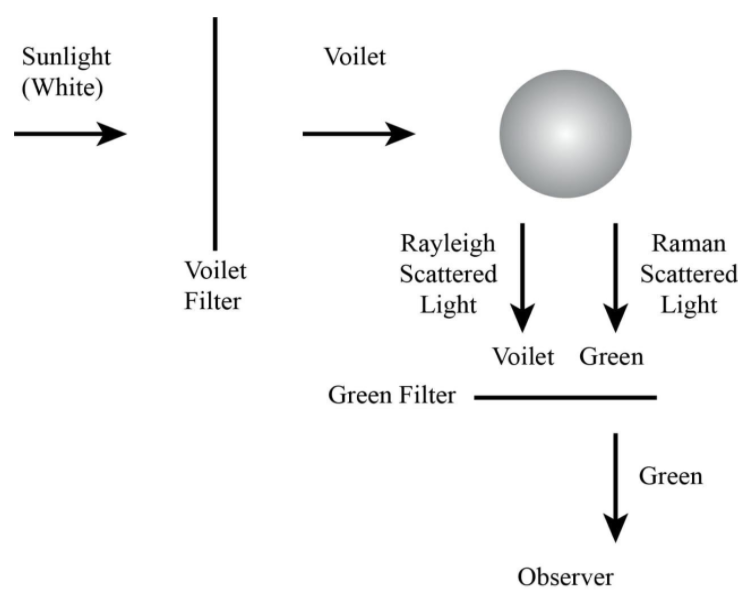
Experiment:
Sir C. V. Raman performed a series of measurements where he focused sunlight on a liquid probe. He used a monochromatic filter (excitation filter) which let only light with a specific wavelength reach the investigation. The measured scattered light showed a broader spectrum with additional wavelengths—a second filter (emission filter) behind the analysis allowed blocking the incident wavelength. The observed residual scattered light could now be clearly distinguished from the incident light.
The observations which Sir Raman made can be explained by the fact that photons which are not absorbed by the probe will be scattered. In “UV-Vis” absorption spectroscopy, electrons in the ground state are excited to a so-called excited electronic state. For this, the photon energy (depending on the wavelength) has to match the difference in the energy states. As a result, those absorbed wavelengths cannot be found in the transmitting light. When light is scattered, electrons are also excited from their ground state. However, the photon energy does not have to be resonant. Molecules can be excited to a virtual energy state. Scattered light itself can be distinguished between elastic and inelastic scattering. The central part scatters elastic, which means that the energy (i.e. wavelength) of the incident light is equal to the emitted light. This phenomenon is referred to as Rayleigh scattering.
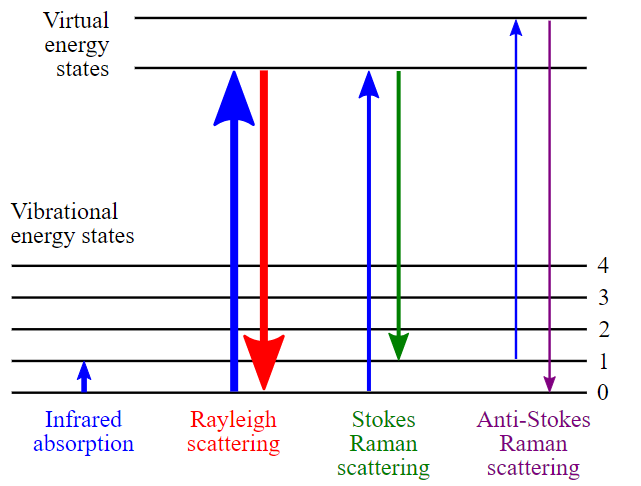
Inelastic scattering is often further distinguished between two different forms, counting on the energy level of the molecule .In case one, the molecule is initially in its state. After excitation, the molecule falls back to a vibrational energy level above the ground state. As a result, the emitted photon has less energy than before, and the scattered light will shift to a higher wavelength.
This effect is Stokes -Raman- scattering. The second type of inelastic scattering assumes that the molecule is already in a higher vibrational state. After excitation, the photon falls back to the molecule’s ground state. The emitted photon has higher energy than the wavelength shifts to lower values.
This effect is called Anti- Stokes- Raman scattering. Anti-Stokes-Raman scattering is usually weaker than Stokes-Raman scattering as most molecules are initially in their state. Hence Stokes -Raman scattering is mainly measured in Raman spectroscopy.
Uses of Raman Effect:
Raman analysis is one of the few techniques which can provide essential information, easily and quickly, detailing the chemical composition and the structure of the investigated materials. Raman spectrometers also detect spin waves (magnons) in semi-magnetic crystals. As for photons, spin waves with small wave numbers (near zero that means at the Centrum of the Brillouin zone) are only detected.
Note: Raman Effect is very weak and cannot be used for metal and alloys; also the impurities of the sample can be hidden by Raman spectrum. A sample heating through the laser radiation can destroy the sample or cover the Raman spectrum.
Complete step by step answer:
When a light beam is travelled through the substance, the light is reflected by the molecules of the essence. It means that the particles of the substance absorb photons of the light energy and subsequently emit light. In this process, the frequency of the absorbed and emitted light will exactly be the same. Thus the light coming out of the substance will have the same energy as that of the incident light; therefore, there is no energy loss, which is called Rayleigh scattering of light. But, Sir C. V. Raman observed a minimal volume (1 in 10 million parts) of the scattered light has a slightly different wavelength compared to the incident light wavelength. This change in the wavelength of the light beam is called a Raman Effect. It forms an essential part of spectroscopy. This happens due to some absorption of energy by molecules.

Experiment:
Sir C. V. Raman performed a series of measurements where he focused sunlight on a liquid probe. He used a monochromatic filter (excitation filter) which let only light with a specific wavelength reach the investigation. The measured scattered light showed a broader spectrum with additional wavelengths—a second filter (emission filter) behind the analysis allowed blocking the incident wavelength. The observed residual scattered light could now be clearly distinguished from the incident light.
The observations which Sir Raman made can be explained by the fact that photons which are not absorbed by the probe will be scattered. In “UV-Vis” absorption spectroscopy, electrons in the ground state are excited to a so-called excited electronic state. For this, the photon energy (depending on the wavelength) has to match the difference in the energy states. As a result, those absorbed wavelengths cannot be found in the transmitting light. When light is scattered, electrons are also excited from their ground state. However, the photon energy does not have to be resonant. Molecules can be excited to a virtual energy state. Scattered light itself can be distinguished between elastic and inelastic scattering. The central part scatters elastic, which means that the energy (i.e. wavelength) of the incident light is equal to the emitted light. This phenomenon is referred to as Rayleigh scattering.

Inelastic scattering is often further distinguished between two different forms, counting on the energy level of the molecule .In case one, the molecule is initially in its state. After excitation, the molecule falls back to a vibrational energy level above the ground state. As a result, the emitted photon has less energy than before, and the scattered light will shift to a higher wavelength.
This effect is Stokes -Raman- scattering. The second type of inelastic scattering assumes that the molecule is already in a higher vibrational state. After excitation, the photon falls back to the molecule’s ground state. The emitted photon has higher energy than the wavelength shifts to lower values.
This effect is called Anti- Stokes- Raman scattering. Anti-Stokes-Raman scattering is usually weaker than Stokes-Raman scattering as most molecules are initially in their state. Hence Stokes -Raman scattering is mainly measured in Raman spectroscopy.
Uses of Raman Effect:
Raman analysis is one of the few techniques which can provide essential information, easily and quickly, detailing the chemical composition and the structure of the investigated materials. Raman spectrometers also detect spin waves (magnons) in semi-magnetic crystals. As for photons, spin waves with small wave numbers (near zero that means at the Centrum of the Brillouin zone) are only detected.
Note: Raman Effect is very weak and cannot be used for metal and alloys; also the impurities of the sample can be hidden by Raman spectrum. A sample heating through the laser radiation can destroy the sample or cover the Raman spectrum.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




