
Destructive distillation of coal leads to the formation of!
A) Wood
B) Kerosene
C) Ammonia liquor
D) Charcoal
Answer
528.1k+ views
Hint: Coal when heated without air doesn’t burn but produces many by-products. The products of destructive distillation are different from that of combustion.
Complete step by step answer:
Destructive distillation is the process of decomposing a material by heating in a closed container in an inert atmosphere and collecting off the volatile constituents. Destructive distillation of coal produces many by-products. In the process, heated coal turns into coke, ammonia dissolves in water to form ammonia liquor and coal gas and coal tar are also produced. The apparatus for destructive distillation used in laboratory is shown below:
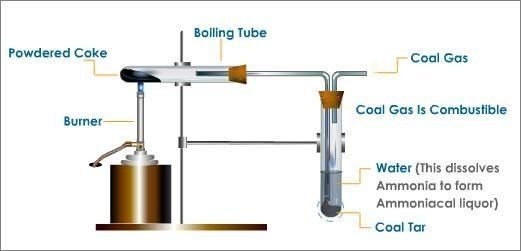
Process of destructive distillation:
In this process, coal is taken in a test tube and heated. Coal breaks down to produce coke, coal tar, ammonia and coal gas. Coal tar is collected at the bottom of the other test tube and coal gas escapes out of it. Ammonia is absorbed in water which forms ammonia liquor \[N{H_4}OH\]. The residue left in the parent test tube is coke.
Hence, the correct answer is (C) ammonia liquor
Additional information: Coal is a very important fossil fuel. It has a variety of uses:
It is an important means of electricity generation.
It is also used in steel production.
It is also used in filters for water and air purification and in kidney dialysis machines.
Note: A student might mistake it for the combustion of coal. Combustion is the burning of a substance in the presence of Oxygen. The products of combustion of coal are CO (Carbon Monoxide) and \[C{O_2}\] (Carbon Dioxide).
Complete step by step answer:
Destructive distillation is the process of decomposing a material by heating in a closed container in an inert atmosphere and collecting off the volatile constituents. Destructive distillation of coal produces many by-products. In the process, heated coal turns into coke, ammonia dissolves in water to form ammonia liquor and coal gas and coal tar are also produced. The apparatus for destructive distillation used in laboratory is shown below:

Process of destructive distillation:
In this process, coal is taken in a test tube and heated. Coal breaks down to produce coke, coal tar, ammonia and coal gas. Coal tar is collected at the bottom of the other test tube and coal gas escapes out of it. Ammonia is absorbed in water which forms ammonia liquor \[N{H_4}OH\]. The residue left in the parent test tube is coke.
Hence, the correct answer is (C) ammonia liquor
Additional information: Coal is a very important fossil fuel. It has a variety of uses:
It is an important means of electricity generation.
It is also used in steel production.
It is also used in filters for water and air purification and in kidney dialysis machines.
Note: A student might mistake it for the combustion of coal. Combustion is the burning of a substance in the presence of Oxygen. The products of combustion of coal are CO (Carbon Monoxide) and \[C{O_2}\] (Carbon Dioxide).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




