
What is the difference between condensation and addition polymerization?
A) In addition polymerization two monomers are added.
B) A polymer and byproduct is formed in condensation polymerization
C) Nylon-6,6 is an example of condensation polymerization
D) All of the above
Answer
521.1k+ views
Hint: We need to know that addition polymerization can be explained as the name says addition, it means we are adding something to get something, whereas in condensation polymerization as the name says something is condensed to give a product. We must need to know how to calculate molecular weight as it is then easy to find about addition and condensation polymerization.
Complete answer:
Let’s study about addition and condensation polymerization in brief:
In addition to polymerization, the monomer must have a multiple bond in it. For example: Acetylene molecule can show addition polymerization
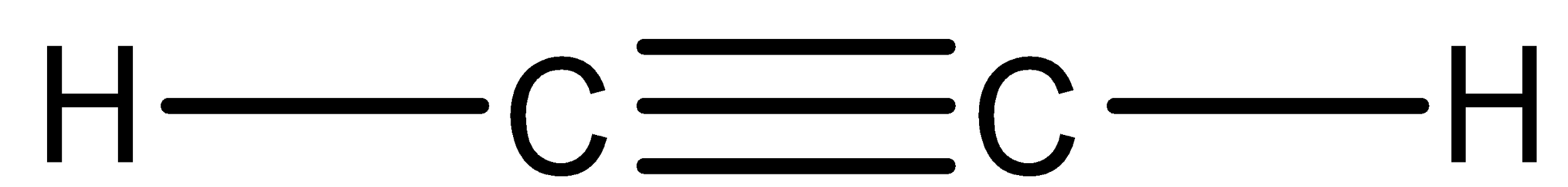
Whereas in condensation polymerization, there must have different or identical functional groups. For example: Glycol
A polymer is formed by adding two or more monomers and no other by product is formed in addition to polymerization whereas in condensation polymerization both byproduct and polymer are formed.
The molecular weight of polymer formed in addition to polymerization is a multiple of the molecular weight of monomer but it is not true in case of condensation polymerization.
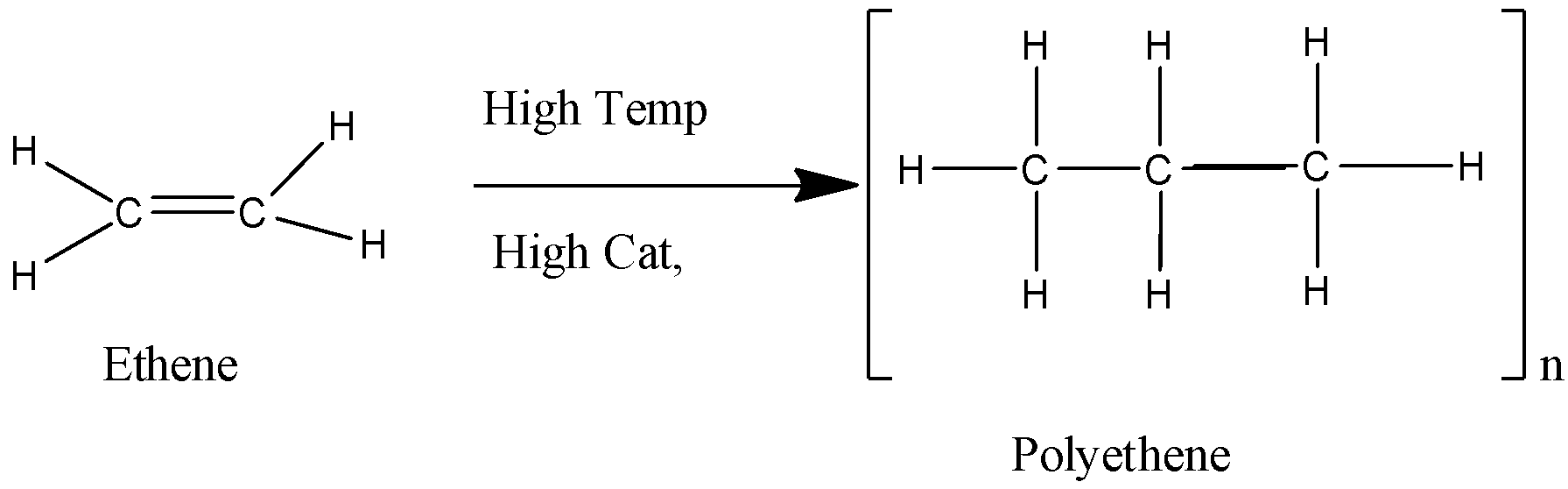
We can draw the addition polymerization reaction as:
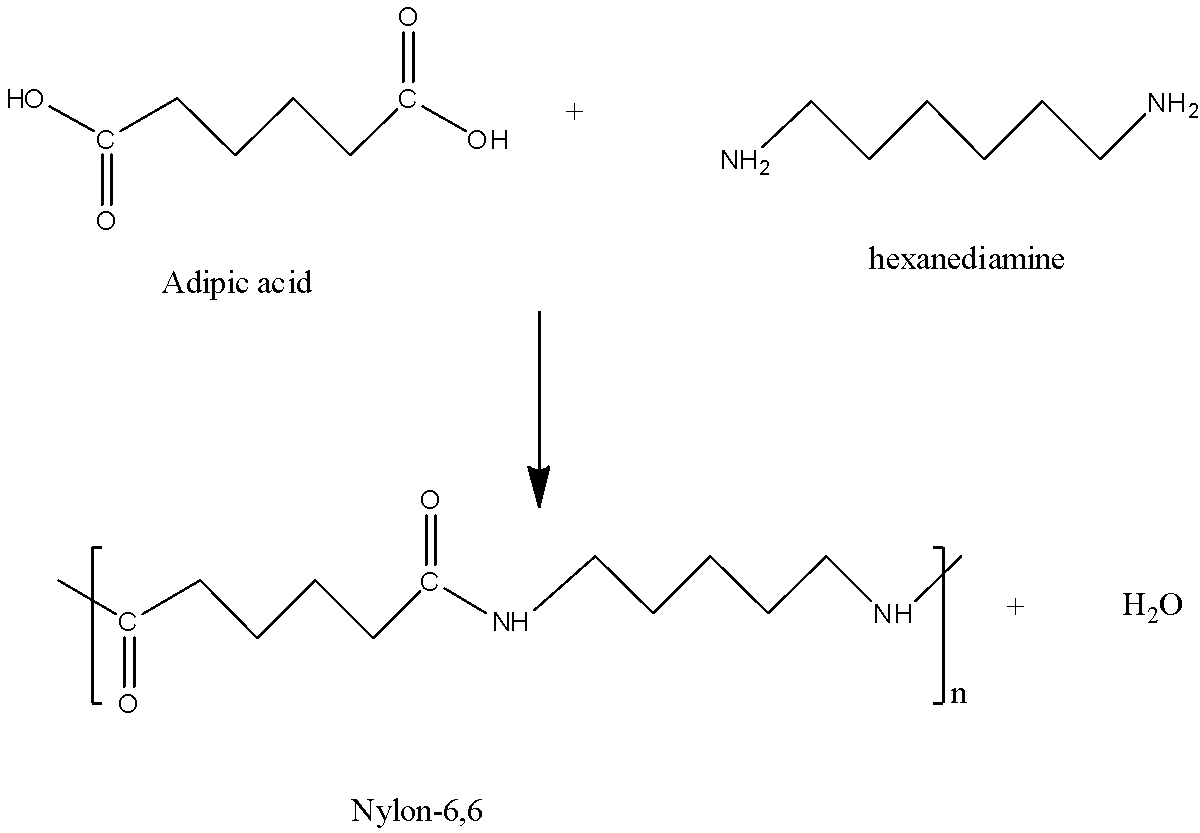
We can draw the condensation polymerization reaction as,
Option A) this option can be correct as addition polymerization involves two monomers.
Option B) This option can be correct as in condensation polymerization a polymer and byproduct is formed as explained above.
Option C) this option can be correct as Nylon-6,6 is an example of condensation polymerization.
Option D) this is a correct option as all the options given above are correct.
Note:
We have to remember that the addition polymerization does not involve the formation of byproduct, as you can see in the above given example only polymer is formed whereas in condensation polymerization both polymer and byproduct is formed as shown in the example above. Water and nylon-6,6 is formed while condensing adipic acid and hexanediamine.
Complete answer:
Let’s study about addition and condensation polymerization in brief:
In addition to polymerization, the monomer must have a multiple bond in it. For example: Acetylene molecule can show addition polymerization
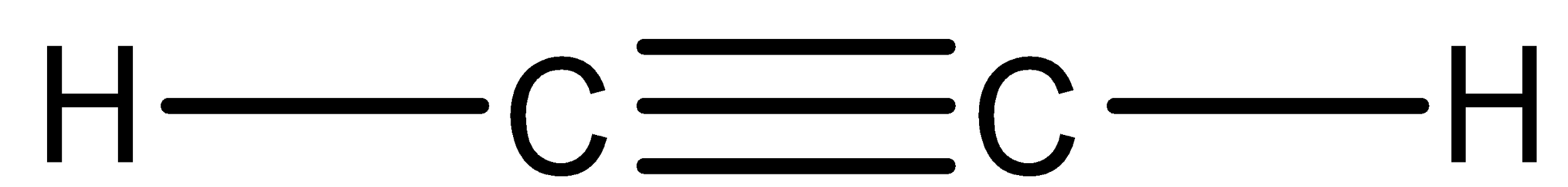
Whereas in condensation polymerization, there must have different or identical functional groups. For example: Glycol
A polymer is formed by adding two or more monomers and no other by product is formed in addition to polymerization whereas in condensation polymerization both byproduct and polymer are formed.
The molecular weight of polymer formed in addition to polymerization is a multiple of the molecular weight of monomer but it is not true in case of condensation polymerization.
We can draw the addition polymerization reaction as:

We can draw the condensation polymerization reaction as,

Option A) this option can be correct as addition polymerization involves two monomers.
Option B) This option can be correct as in condensation polymerization a polymer and byproduct is formed as explained above.
Option C) this option can be correct as Nylon-6,6 is an example of condensation polymerization.
Option D) this is a correct option as all the options given above are correct.
Note:
We have to remember that the addition polymerization does not involve the formation of byproduct, as you can see in the above given example only polymer is formed whereas in condensation polymerization both polymer and byproduct is formed as shown in the example above. Water and nylon-6,6 is formed while condensing adipic acid and hexanediamine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




