
Distinguish between metals, insulators, and semiconductors on the basis of band theory.
Answer
516.3k+ views
Hint: The valence band of insulators remains whole of electrons. The conduction band remains empty. The forbidden energy gap is most widespread. Crossing the forbidden gap requires enormous energy. There is an overlapping of the valence band and the conduction band in metals. There is no forbidden energy gap. The resistance of the conductor is meagre. In a semiconductor, the band gap is smaller. With the rise of temperature, the electrical conductivity of semiconductors increases. So, the electricity can move quickly through the conductors.
Complete step-by-step solution:
Metals: In metals, the conduction band is either somewhat filled, or the valence band is partly empty. Some electrons act as free electrons as they move to higher energy levels by obtaining energy beyond the Fermi level in the conduction band. There is no restricted-energy gap in the metals. Since there is no forbidden gap, the electron numbers available for the conduction increase the material's conductivity. Metals work as a conductor because of the motion of the free electrons when a minute amount of electric current is implemented. Silver, Aluminium, etc., are good conductors.
Insulators: In insulators, the valence band is occupied while the conduction band is blank. This ends in a large energy gap. Since the energy passage between the conduction band and the valence band is higher, there is no shift of electrons from the valence band to the conduction band. Mica, ebonite, glass, etc., are examples of insulators.
Semiconductor: In a semiconductor, the valence band is loaded with electrons while the conduction band is blank. The energy gap separating the bands is smaller. For electrons to escape from the valence band to the conduction band, the temperature of the room must be reserved. If the temperature is zero Kelvin, there is no motion of electrons from the valence band to the conduction band.
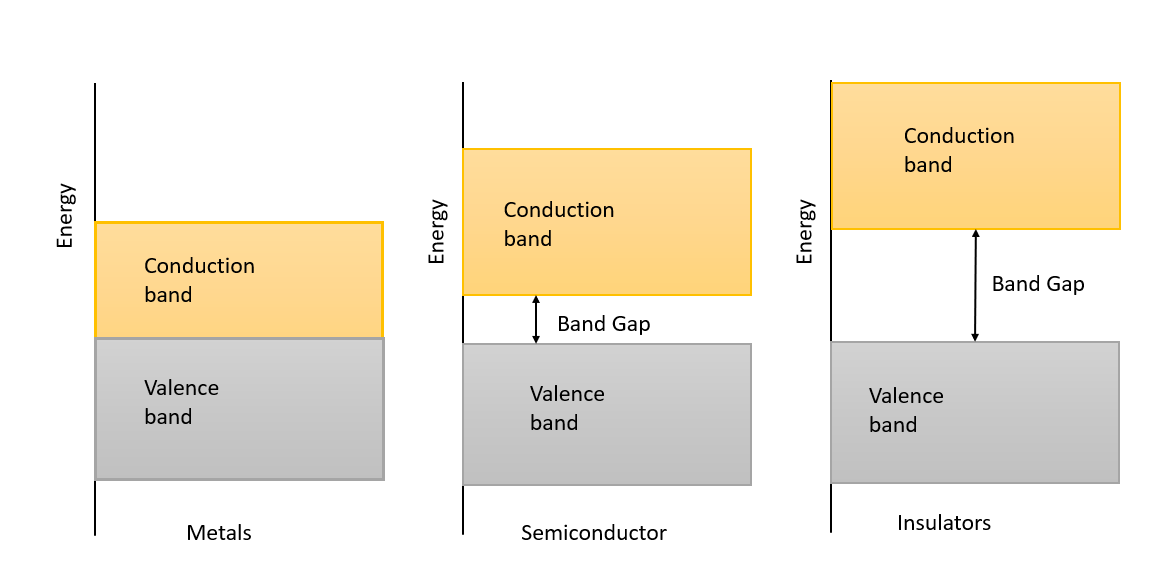
Note: There is a small gap between conduction and valence band in metals. The conduction band is completely occupied. On applying a small electric field, metals can carry current. However, there is a large gap between conduction and valence band. The conduction band is empty. When an electric field is applied on insulators, electrons find it challenging to reach the conduction band. Therefore, no current flows in insulators.
Complete step-by-step solution:
Metals: In metals, the conduction band is either somewhat filled, or the valence band is partly empty. Some electrons act as free electrons as they move to higher energy levels by obtaining energy beyond the Fermi level in the conduction band. There is no restricted-energy gap in the metals. Since there is no forbidden gap, the electron numbers available for the conduction increase the material's conductivity. Metals work as a conductor because of the motion of the free electrons when a minute amount of electric current is implemented. Silver, Aluminium, etc., are good conductors.
Insulators: In insulators, the valence band is occupied while the conduction band is blank. This ends in a large energy gap. Since the energy passage between the conduction band and the valence band is higher, there is no shift of electrons from the valence band to the conduction band. Mica, ebonite, glass, etc., are examples of insulators.
Semiconductor: In a semiconductor, the valence band is loaded with electrons while the conduction band is blank. The energy gap separating the bands is smaller. For electrons to escape from the valence band to the conduction band, the temperature of the room must be reserved. If the temperature is zero Kelvin, there is no motion of electrons from the valence band to the conduction band.

Note: There is a small gap between conduction and valence band in metals. The conduction band is completely occupied. On applying a small electric field, metals can carry current. However, there is a large gap between conduction and valence band. The conduction band is empty. When an electric field is applied on insulators, electrons find it challenging to reach the conduction band. Therefore, no current flows in insulators.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




