
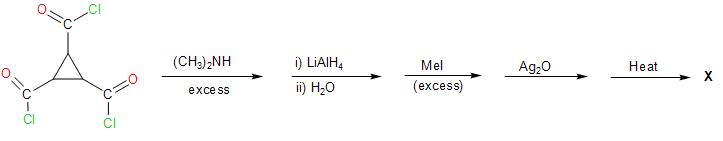
What is the double bond equivalent of X (final product) in the above sequence?
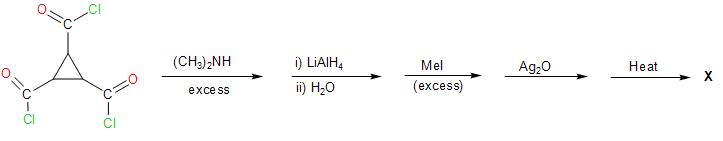
Answer
577.8k+ views
Hint: In order to determine the double bond equivalent, we will look for the double bond and the ring present in the final product formed by the given reaction sequence.
Complete step by step answer:
In the given reaction sequence, the reactant cyclopropane-1,2,3-tricarbonyl chloride on reaction with substituted amine, that is, dimethyl amine ${{(C{{H}_{3}})}_{2}}NH$ which is a strong base. The nucleophilic acyl substitution reaction (through ${{S}_{N}}2$ mechanism) takes place as the base acts as a strong nucleophile and attacks the carbonyl carbon atom. The base abstracts a proton, leaving behind ${{(C{{H}_{3}})}_{2}}N-$ group attached to the carbonyl carbon. The chloride leaves being a better leaving group. We thus, get a tertiary amide group formed.
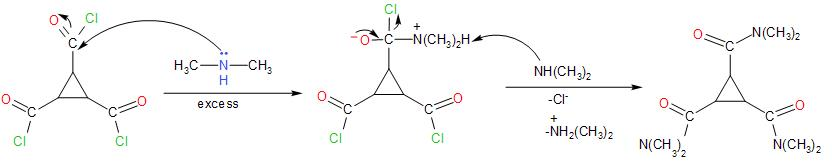
Further, the reaction with lithium aluminium hydride leads to the reduction of the carbonyl group present in the compound to ${{3}^{\circ }}$ amine.
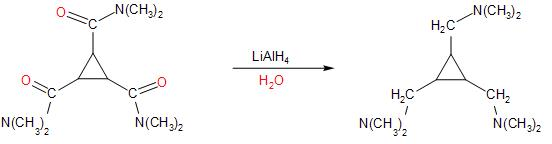
Followed by the reaction with methyl iodide, leads to the alkylation of the tertiary amine formed in the above step. The lone pair of electrons on the nitrogen atom of the amine causes nucleophilic attack on the methyl group of $C{{H}_{3}}-I$.
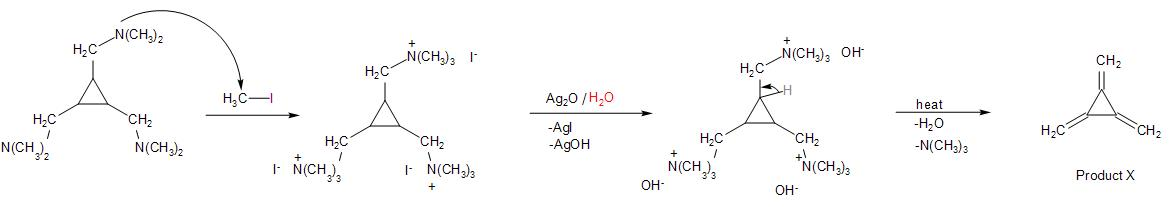
The alkyl ammonium salt reacts with silver oxide and water, such that it produces silver iodide and silver hydroxide. The $O{{H}^{-}}$ from the water, abstracts protons for the adjacent carbon atom, forming a double bond as the tertiary amine leaves the compound.
Therefore, the double bond equivalent in the product formed involves three pi-bonds and a ring. So, the degree of unsaturation is 4.
Note: The double bond equivalent accounts for the degree of unsaturation in the system, that is the presence of a double bond or a ring system.
Complete step by step answer:
In the given reaction sequence, the reactant cyclopropane-1,2,3-tricarbonyl chloride on reaction with substituted amine, that is, dimethyl amine ${{(C{{H}_{3}})}_{2}}NH$ which is a strong base. The nucleophilic acyl substitution reaction (through ${{S}_{N}}2$ mechanism) takes place as the base acts as a strong nucleophile and attacks the carbonyl carbon atom. The base abstracts a proton, leaving behind ${{(C{{H}_{3}})}_{2}}N-$ group attached to the carbonyl carbon. The chloride leaves being a better leaving group. We thus, get a tertiary amide group formed.

Further, the reaction with lithium aluminium hydride leads to the reduction of the carbonyl group present in the compound to ${{3}^{\circ }}$ amine.

Followed by the reaction with methyl iodide, leads to the alkylation of the tertiary amine formed in the above step. The lone pair of electrons on the nitrogen atom of the amine causes nucleophilic attack on the methyl group of $C{{H}_{3}}-I$.

The alkyl ammonium salt reacts with silver oxide and water, such that it produces silver iodide and silver hydroxide. The $O{{H}^{-}}$ from the water, abstracts protons for the adjacent carbon atom, forming a double bond as the tertiary amine leaves the compound.
Therefore, the double bond equivalent in the product formed involves three pi-bonds and a ring. So, the degree of unsaturation is 4.
Note: The double bond equivalent accounts for the degree of unsaturation in the system, that is the presence of a double bond or a ring system.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




