
How to draw Lewis structure for ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$ and ${\text{NO}}_{\text{2}}^{\text{ - }}$.
Answer
560.7k+ views
Hint: For drawing the Lewis structure of any compound or molecule we have to know about the number of valence electrons present in that compound and the valency of each atom present in that compound.
Complete step by step answer:
For drawing the Lewis structure of Sulfate ion (${\text{SO}}_{\text{4}}^{{\text{2 - }}}$) we have to follow the following steps:
-First we calculate the total number of valence electrons present in the sulfate ion and for this we will add all the valence electrons of each atom and charge present on that molecule.
-Valence electrons on sulfur atom (${\text{S}}$) = $6$
Valence electrons on oxygen atom (${\text{O}}$) = $6$
Electrons on ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$molecule due to charge = $2$
Total electrons on ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$ molecule = $6 + \left( {4 \times 6} \right) + 2 = 32$
-Now we construct a basic structure of ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$ molecule by taking sulfur atom as a central atom because it is less electro-positive as compared to oxygen atom.
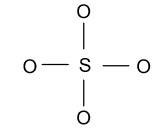
-In the above figure eight electrons are involved in the formation of four bonds and then we have $24$ electrons left. Now we will distribute these ${\text{24}}{{\text{e}}^{\text{ - }}}$ among four oxygen atoms as follow:
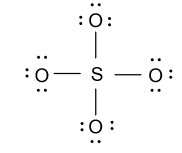
-As we know that sulfur has six valence electrons but in the above structure only four electrons are involved so for satisfying the valency of sulfur, we will convert two lone pairs of electrons from two different oxygen atoms to double bond as follow:
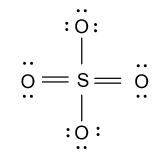
Hence Lewis structure of sulfate ion is shown as below:
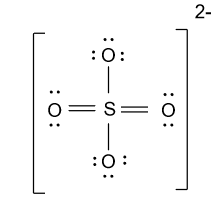
For drawing the Lewis structure of Nitrite ion (${\text{NO}}_2^{\text{ - }}$) we have to follow the following steps:
-First we calculate the total number of valence electrons present in the nitrite ion and for this we will add all the valence electrons of each atom and charge present on that molecule.
-Valence electrons on nitrogen atom (${\text{N}}$) = $5$
Valence electrons on oxygen atom (${\text{O}}$) = $6$
Electrons on ${\text{NO}}_2^{\text{ - }}$molecule due to charge = $1$
Total electrons on ${\text{NO}}_2^{\text{ - }}$molecule = $5 + \left( {2 \times 6} \right) + 1 = 18$
-Now we construct a basic structure of ${\text{NO}}_2^{\text{ - }}$ molecule by taking nitrogen atom as a central atom because it is less electro-positive as compared to oxygen atom.
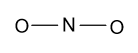
-In the above figure four electrons are involved in the formation of two bonds and then we have $18$ electrons left. Now we will distribute these ${\text{18}}{{\text{e}}^{\text{ - }}}$ among two oxygen atoms and one nitrogen atom as follow:
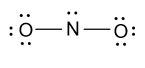
-As we know that nitrogen has five valence electrons but in the above structure only four electrons are involved so for satisfying the valency of nitrogen, we will convert one lone pair of electrons from one oxygen atoms to double bond as follow

Hence Lewis structure of nitrite ion is shown as below:
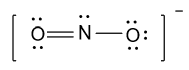
Note: Here some of you may draw the wrong structure if you didn’t count the charge present on a molecule as an electron during calculating the total number of valence electrons of the given compound.
Complete step by step answer:
For drawing the Lewis structure of Sulfate ion (${\text{SO}}_{\text{4}}^{{\text{2 - }}}$) we have to follow the following steps:
-First we calculate the total number of valence electrons present in the sulfate ion and for this we will add all the valence electrons of each atom and charge present on that molecule.
-Valence electrons on sulfur atom (${\text{S}}$) = $6$
Valence electrons on oxygen atom (${\text{O}}$) = $6$
Electrons on ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$molecule due to charge = $2$
Total electrons on ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$ molecule = $6 + \left( {4 \times 6} \right) + 2 = 32$
-Now we construct a basic structure of ${\text{SO}}_{\text{4}}^{{\text{2 - }}}$ molecule by taking sulfur atom as a central atom because it is less electro-positive as compared to oxygen atom.

-In the above figure eight electrons are involved in the formation of four bonds and then we have $24$ electrons left. Now we will distribute these ${\text{24}}{{\text{e}}^{\text{ - }}}$ among four oxygen atoms as follow:

-As we know that sulfur has six valence electrons but in the above structure only four electrons are involved so for satisfying the valency of sulfur, we will convert two lone pairs of electrons from two different oxygen atoms to double bond as follow:

Hence Lewis structure of sulfate ion is shown as below:

For drawing the Lewis structure of Nitrite ion (${\text{NO}}_2^{\text{ - }}$) we have to follow the following steps:
-First we calculate the total number of valence electrons present in the nitrite ion and for this we will add all the valence electrons of each atom and charge present on that molecule.
-Valence electrons on nitrogen atom (${\text{N}}$) = $5$
Valence electrons on oxygen atom (${\text{O}}$) = $6$
Electrons on ${\text{NO}}_2^{\text{ - }}$molecule due to charge = $1$
Total electrons on ${\text{NO}}_2^{\text{ - }}$molecule = $5 + \left( {2 \times 6} \right) + 1 = 18$
-Now we construct a basic structure of ${\text{NO}}_2^{\text{ - }}$ molecule by taking nitrogen atom as a central atom because it is less electro-positive as compared to oxygen atom.
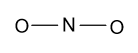
-In the above figure four electrons are involved in the formation of two bonds and then we have $18$ electrons left. Now we will distribute these ${\text{18}}{{\text{e}}^{\text{ - }}}$ among two oxygen atoms and one nitrogen atom as follow:
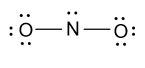
-As we know that nitrogen has five valence electrons but in the above structure only four electrons are involved so for satisfying the valency of nitrogen, we will convert one lone pair of electrons from one oxygen atoms to double bond as follow

Hence Lewis structure of nitrite ion is shown as below:
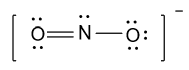
Note: Here some of you may draw the wrong structure if you didn’t count the charge present on a molecule as an electron during calculating the total number of valence electrons of the given compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




