
How do you draw the Lewis structure for the sulfate ion, $SO_4^{2 - }$?
Answer
558k+ views
Hint: In sulfate ion, the central atom sulphur is bonded with the four oxygen atoms by a single bond. The overall charge of the sulfate ion is -2. The total valence electrons of sulfate ions is 32.
Complete step by step answer:
Lewis structure is defined as the representation for the arrangement of valence electrons surrounding each atom present in the molecule.
The sulfate ion is the oxyanion of sulfur where the oxidation state of sulfur is +6 and the overall charge on the sulfate ion is -2.
The sulfur and oxygen atom are present in the VI group of the periodic table. So, the total number of valence electrons present in sulfur and oxygen is 6.
Total valence electrons in sulfur = 6
As four oxygen atoms are present in the sulfate ion. So the total valence electrons on oxygen will be
$ \Rightarrow 4 \times 6 = 24$
As, -2 charge is present on the sulfate ion, two more electrons are added
So, the total valence electrons will be
$ \Rightarrow 6 + 24 + 2 = 32$
The total electron pairs are calculated by dividing the total valence electrons by 2.
$ \Rightarrow $total electron pairs = \[\dfrac{{32}}{2}\]
$ \Rightarrow $total electron pairs = 16
The Lewis structure of sulfate ion is shown below.
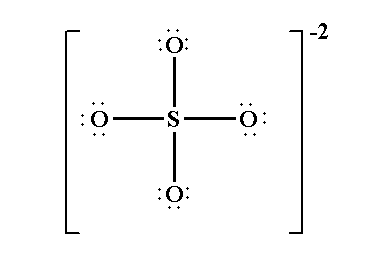
Out of 16 electron pairs, the four electron pairs form four bond S-O and the remaining 12 electron pairs form lone pairs at the four oxygen atoms.
Note:
In sulfate ion, each oxygen atom carries -1 charge and the central sulfur atom carries +2 charge. So, $(( - 1)(4) + ( + 2)) = - 2$. Therefore, the overall charge of sulfate ions is -2.
Complete step by step answer:
Lewis structure is defined as the representation for the arrangement of valence electrons surrounding each atom present in the molecule.
The sulfate ion is the oxyanion of sulfur where the oxidation state of sulfur is +6 and the overall charge on the sulfate ion is -2.
The sulfur and oxygen atom are present in the VI group of the periodic table. So, the total number of valence electrons present in sulfur and oxygen is 6.
Total valence electrons in sulfur = 6
As four oxygen atoms are present in the sulfate ion. So the total valence electrons on oxygen will be
$ \Rightarrow 4 \times 6 = 24$
As, -2 charge is present on the sulfate ion, two more electrons are added
So, the total valence electrons will be
$ \Rightarrow 6 + 24 + 2 = 32$
The total electron pairs are calculated by dividing the total valence electrons by 2.
$ \Rightarrow $total electron pairs = \[\dfrac{{32}}{2}\]
$ \Rightarrow $total electron pairs = 16
The Lewis structure of sulfate ion is shown below.

Out of 16 electron pairs, the four electron pairs form four bond S-O and the remaining 12 electron pairs form lone pairs at the four oxygen atoms.
Note:
In sulfate ion, each oxygen atom carries -1 charge and the central sulfur atom carries +2 charge. So, $(( - 1)(4) + ( + 2)) = - 2$. Therefore, the overall charge of sulfate ions is -2.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




