
Draw the resonating structures of aniline.
Answer
528k+ views
Hint:The resonance structures of aniline are drawn by first displacing the lone pair of electrons on the nitrogen to the bond between C and N. This results in formation of a double bond between C and N with N getting a positive charge due to the donation of electrons.
Complete answer:
- Aniline has the chemical formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$ . It is an organic compound that consists of a phenyl group attached to an amine group.
- Aniline is considered to be the simplest aromatic amine.
- Aniline has the odour of a rotten fish.
- Aniline has 5 resonating structures. The resonance structures of aniline are
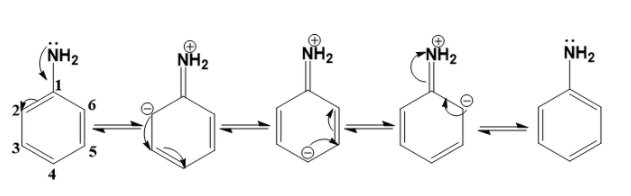
- The resonance structures of aniline are obtained by first displacing the lone pair of electrons on the nitrogen to the C –N bond. This results in the formation of a double bond between C and N with N getting a positive charge due to the donation of electrons.
- The valency of C is 4. This means that C can form 4 bonds. As C-1 already had 4 bonds, the electrons on the C=C pi bond move to the adjacent C-2 atom and the C-2 atom gets a negative charge. This is how the second structure is formed.
- Similarly, the negative on the C-2 atom will displace to the adjacent bond. The pi electrons on the adjacent bond then move to the C-4 atom and it gets a negative charge. This is how the third structure is formed.
- Next, the negative on the C-4 atom will displace to the adjacent bond. The pi electrons on the adjacent bond then move to C-6 atom and it gets a negative charge. This is how the fourth structure is formed.
- Finally, the negative on the C-6 atom will displace to the adjacent bond. The pi electrons on the C=N bond then move to N atom. This is how the fifth structure is formed.
- Hence, there are five resonating structures of aniline.
Additional Information:
- Aniline has a wide range of uses.
- It is used in rubber accelerators and antioxidants.
- Aniline is also an ingredient in dyes
- It is used in pharmaceuticals and petroleum refining.
- It is used in the industrial production of diphenylamine, herbicides and fungicides.
Note:
Be careful while drawing the resonating structures and remember that only pi electrons and lone pairs can be delocalised. Only five structures of aniline exist in its resonating form.
Complete answer:
- Aniline has the chemical formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$ . It is an organic compound that consists of a phenyl group attached to an amine group.
- Aniline is considered to be the simplest aromatic amine.
- Aniline has the odour of a rotten fish.
- Aniline has 5 resonating structures. The resonance structures of aniline are

- The resonance structures of aniline are obtained by first displacing the lone pair of electrons on the nitrogen to the C –N bond. This results in the formation of a double bond between C and N with N getting a positive charge due to the donation of electrons.
- The valency of C is 4. This means that C can form 4 bonds. As C-1 already had 4 bonds, the electrons on the C=C pi bond move to the adjacent C-2 atom and the C-2 atom gets a negative charge. This is how the second structure is formed.
- Similarly, the negative on the C-2 atom will displace to the adjacent bond. The pi electrons on the adjacent bond then move to the C-4 atom and it gets a negative charge. This is how the third structure is formed.
- Next, the negative on the C-4 atom will displace to the adjacent bond. The pi electrons on the adjacent bond then move to C-6 atom and it gets a negative charge. This is how the fourth structure is formed.
- Finally, the negative on the C-6 atom will displace to the adjacent bond. The pi electrons on the C=N bond then move to N atom. This is how the fifth structure is formed.
- Hence, there are five resonating structures of aniline.
Additional Information:
- Aniline has a wide range of uses.
- It is used in rubber accelerators and antioxidants.
- Aniline is also an ingredient in dyes
- It is used in pharmaceuticals and petroleum refining.
- It is used in the industrial production of diphenylamine, herbicides and fungicides.
Note:
Be careful while drawing the resonating structures and remember that only pi electrons and lone pairs can be delocalised. Only five structures of aniline exist in its resonating form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




