
EDTA is hexadentate ligand and is used to remove hardness in water.
A.True
B.False
Answer
583.5k+ views
Hint: EDTA is Hexadentate ligand. It forms by the complex with central metal and ligands. Hexadentate ligand combines with the central atom with six bonds. It forms very stable complexes with most of the transition metals. EDTA forms by the nitrogen and oxygen atoms, where oxygen contains negatively charged..
Complete step by step answer:
EDTA is a hexadentate ligand which is able to donate electrons from six sites. It is formed by two nitrogens and four oxygen. Here ligand has a number of lone pair electrons to donate the central atom. Many ligands are capable of binding metal by different sites because ligands have lone pair electrons on more than one atom.
The full form of EDTA is ethylene diamine tetra acetic acid. As we know the hardness of water is due to the presence of $M{g^{ + 2}}$ and $C{a^{ + 2}}$ in it. They are in sulphate or carbonate forms in water. EDTA is able to form stable complexes with these ions and estimate by simple titration.
The structure of EDTA is given below, which showed that EDTA is hexadentate ligand,
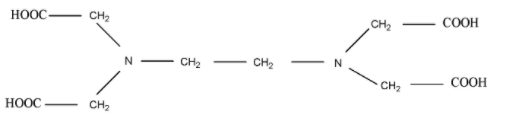
EDTA is also used to remove hardness of water because it forms stable complexes with ions which present in water.
Hence, This statement is true.
Therefore, option (A) is the correct answer.
Note:
EDTA is polyprotic acid which contains four carboxylic groups and two amines with lone pair electrons. It is able to form stable complexes with water to remove its hardness because $M{g^{ + 2}}$ and $C{a^{ + 2}}$ react with this ligand to form complexes.
Complete step by step answer:
EDTA is a hexadentate ligand which is able to donate electrons from six sites. It is formed by two nitrogens and four oxygen. Here ligand has a number of lone pair electrons to donate the central atom. Many ligands are capable of binding metal by different sites because ligands have lone pair electrons on more than one atom.
The full form of EDTA is ethylene diamine tetra acetic acid. As we know the hardness of water is due to the presence of $M{g^{ + 2}}$ and $C{a^{ + 2}}$ in it. They are in sulphate or carbonate forms in water. EDTA is able to form stable complexes with these ions and estimate by simple titration.
The structure of EDTA is given below, which showed that EDTA is hexadentate ligand,

EDTA is also used to remove hardness of water because it forms stable complexes with ions which present in water.
Hence, This statement is true.
Therefore, option (A) is the correct answer.
Note:
EDTA is polyprotic acid which contains four carboxylic groups and two amines with lone pair electrons. It is able to form stable complexes with water to remove its hardness because $M{g^{ + 2}}$ and $C{a^{ + 2}}$ react with this ligand to form complexes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




