
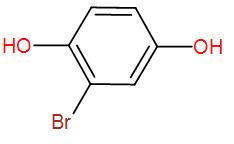
End product of the following reaction is:
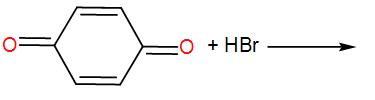
[A]
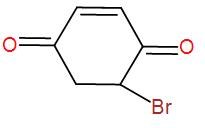
[B]
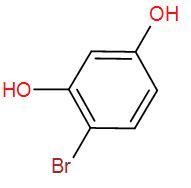
[C]
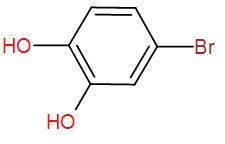
[D]





Answer
591k+ views
Hint: The compound given to us is benzoquinone. In the presence of HBr, it will undergo bromination. On bromination we will get the enol form of the brominated product. It is important to take the resonating structure of the given compound while trying to find out the brominated product.
Complete step by step answer:
The compound given to us is 1, 4- Benzoquinone. It is also known as para-quinone. In presence of hydrogen bromide, it undergoes bromination. We Know that bromination is a reaction in which bromine is introduced inside the molecule that undergoes bromination.
The most common reagent that we use for bromination is hydrogen bromide i.e. HBr.
Here, the question is about the product obtained when 1, 4- Benzoquinone undergoes bromination.
As we can understand from the above discussion, the final product will obviously have a bromine atom in it.
Now, we will discuss the mechanism of the reaction-
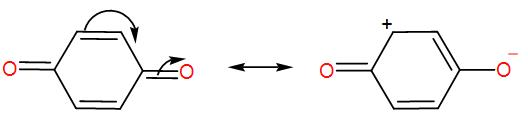
We can write the resonance canonical structure of 1, 4 Benzoquinone as-
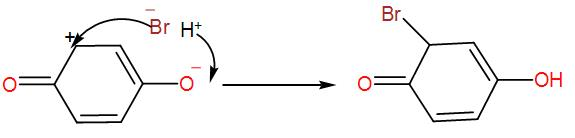
Now, to this we can write the addition of hydrogen bromide-
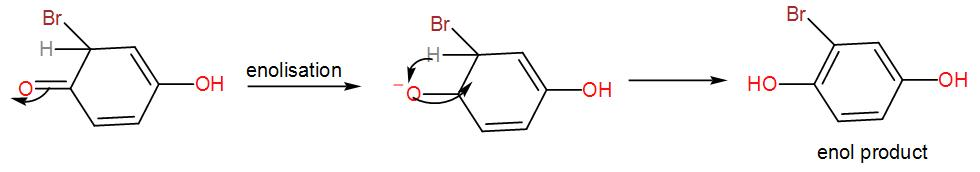
Now, the brominated compound will undergo enolisation reaction i.e. the formation of enol. Enolisation is the process where the –keto group is converted to –enol i.e. the double bonded oxygen atom will become hydroxyl group, –OH . Enolisation of the above product will give us-
Therefore, the final product that we obtain is the enolised product and the name is Bromo-1,4-dihydroxybenzene.
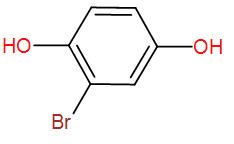
So, the correct answer is “Option D”.
Note: It is important to know here that a compound will be analysed only if it contains an alpha- carbon and at least one hydrogen atom directly attached to the alpha carbon atom. Whether the keto-product is stable or the enol product is stable depends on the type of solvent, hydrogen bonding between the compound and the solvent and also solvation effects.
Here, we get the enol product as it is aromatic and more stable.
Complete step by step answer:
The compound given to us is 1, 4- Benzoquinone. It is also known as para-quinone. In presence of hydrogen bromide, it undergoes bromination. We Know that bromination is a reaction in which bromine is introduced inside the molecule that undergoes bromination.
The most common reagent that we use for bromination is hydrogen bromide i.e. HBr.
Here, the question is about the product obtained when 1, 4- Benzoquinone undergoes bromination.
As we can understand from the above discussion, the final product will obviously have a bromine atom in it.
Now, we will discuss the mechanism of the reaction-
We can write the resonance canonical structure of 1, 4 Benzoquinone as-

Now, to this we can write the addition of hydrogen bromide-

Now, the brominated compound will undergo enolisation reaction i.e. the formation of enol. Enolisation is the process where the –keto group is converted to –enol i.e. the double bonded oxygen atom will become hydroxyl group, –OH . Enolisation of the above product will give us-

Therefore, the final product that we obtain is the enolised product and the name is Bromo-1,4-dihydroxybenzene.

So, the correct answer is “Option D”.
Note: It is important to know here that a compound will be analysed only if it contains an alpha- carbon and at least one hydrogen atom directly attached to the alpha carbon atom. Whether the keto-product is stable or the enol product is stable depends on the type of solvent, hydrogen bonding between the compound and the solvent and also solvation effects.
Here, we get the enol product as it is aromatic and more stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




