
Endosmosis of water occurs, when in comparison with the outer solution the water potential of cell sap is
(a)Higher
(b)Lower
(c)Equal
(d)None of the above
Answer
594k+ views
Hint: The term ‘endo’ means inside or within. Thus endosmosis refers to the movement of the solution inside the cell. The swelling of resin when kept in water, the absorption of water by the plants via roots are some of the examples of the endosmosis process.
Complete answer:
Endosmosis is a process by which the water or the solvent will move inside the cell. Endosmosis will occur when the water potential of the outside environment is greater than the inside environment. In endosmosis, the solute concentration in the external environment will be lesser than the cytosol. Therefore, the movement of water occurs against the concentration gradient. The water enters the cell via the semi-permeable plasma membrane. As more, water is taken from the external environment, the cell will swell and becomes turgid.
Additional Information:
-Osmosis is a process by which the solute molecule moves from the region of higher concentration to the lower concentration (along the concentration gradient) to maintain the same concentration of the solute on both sides. When the solute concentration becomes the same on both sides, then it is said to be in equilibrium.
-Water will move from the area of higher water potential/ less negative water potential to the area where water potential is low/ positive water potential. Having higher water potential means the water will have a larger number of free water molecules than the water having lower water potential.
When cells are kept in a hypotonic solution (solution with lower solute concentration than the cell), endosmosis will occur.
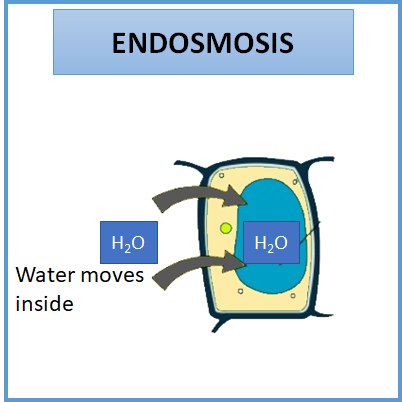
So, the correct answer is ‘Lower’.
Note: One very interesting point is that, when an animal cell such as a red blood cell (RBC) is kept in a hypotonic solution, the cell will initially take up the solution and swell. After some time, the cell bursts. Whereas the plant cell will never burst because of the presence of the cell wall.
Complete answer:
Endosmosis is a process by which the water or the solvent will move inside the cell. Endosmosis will occur when the water potential of the outside environment is greater than the inside environment. In endosmosis, the solute concentration in the external environment will be lesser than the cytosol. Therefore, the movement of water occurs against the concentration gradient. The water enters the cell via the semi-permeable plasma membrane. As more, water is taken from the external environment, the cell will swell and becomes turgid.
Additional Information:
-Osmosis is a process by which the solute molecule moves from the region of higher concentration to the lower concentration (along the concentration gradient) to maintain the same concentration of the solute on both sides. When the solute concentration becomes the same on both sides, then it is said to be in equilibrium.
-Water will move from the area of higher water potential/ less negative water potential to the area where water potential is low/ positive water potential. Having higher water potential means the water will have a larger number of free water molecules than the water having lower water potential.
When cells are kept in a hypotonic solution (solution with lower solute concentration than the cell), endosmosis will occur.

So, the correct answer is ‘Lower’.
Note: One very interesting point is that, when an animal cell such as a red blood cell (RBC) is kept in a hypotonic solution, the cell will initially take up the solution and swell. After some time, the cell bursts. Whereas the plant cell will never burst because of the presence of the cell wall.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




