
Ethanoic acid reacts with ethanol in the presence of conc. ${H_2}S{O_4}$, so as to form a compound and water. The chemical reaction which takes place is called:
(A) dehydration
(B) hydrogenation
(C) esterification
(D) disproportionation
Answer
584.7k+ views
Hint: When primary alcohol is treated with a carboxylic acid in the presence of sulphuric acid a compound which has a sweet smell is formed. The compound produced is called an ester.
Complete step by step solution:
The process of combining ethanoic acid with ethanol to form an ester (RCOOR) and water is called esterification. We can also define it as the chemical reaction resulting in the formation of at least one ester product. Concentrated sulphuric acid is used as a catalyst usually. In some cases, dry hydrogen chloride gas is used as a catalyst but these tend to involve aromatic esters.
The esterification reaction is slow and reversible.
Let us see the mechanism involved in esterification.
The oxygen in the carboxyl group gets protonated to give delocalized carbocation making the carbocation a better electrophile. A proton is transferred to one of the hydroxyl groups. It forms a good leaving group. The oxygen atom of the hydroxyl alcohol group donates a pair of electrons to a carbon atom which makes a $\Pi $ bond by eliminating water. The so formed protonated ester finally loses a proton to give the ester.
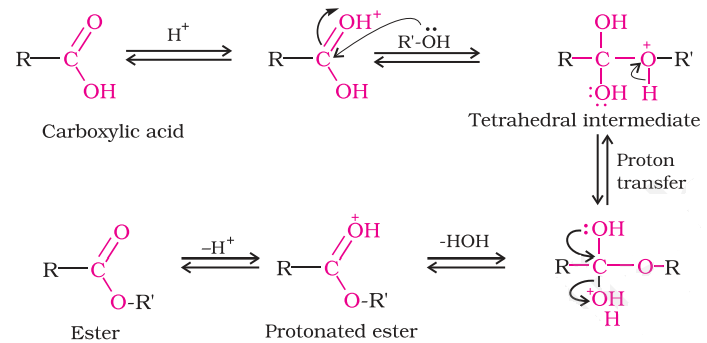
So the correct option is (C) esterification.
Note: Esterification can also happen in two other ways. They are from acid anhydride and alcohol; from acid chloride and alcohol. Ester and water are the products in all three processes. Do not confuse it with disproportionation reaction, as in a disproportionation reaction, a compound with intermediate oxidation state converts into two compounds, one with higher oxidation and other with lower. But here, two compounds combine to give an ester.
Complete step by step solution:
The process of combining ethanoic acid with ethanol to form an ester (RCOOR) and water is called esterification. We can also define it as the chemical reaction resulting in the formation of at least one ester product. Concentrated sulphuric acid is used as a catalyst usually. In some cases, dry hydrogen chloride gas is used as a catalyst but these tend to involve aromatic esters.
The esterification reaction is slow and reversible.
Let us see the mechanism involved in esterification.
The oxygen in the carboxyl group gets protonated to give delocalized carbocation making the carbocation a better electrophile. A proton is transferred to one of the hydroxyl groups. It forms a good leaving group. The oxygen atom of the hydroxyl alcohol group donates a pair of electrons to a carbon atom which makes a $\Pi $ bond by eliminating water. The so formed protonated ester finally loses a proton to give the ester.

So the correct option is (C) esterification.
Note: Esterification can also happen in two other ways. They are from acid anhydride and alcohol; from acid chloride and alcohol. Ester and water are the products in all three processes. Do not confuse it with disproportionation reaction, as in a disproportionation reaction, a compound with intermediate oxidation state converts into two compounds, one with higher oxidation and other with lower. But here, two compounds combine to give an ester.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




