
Ethyl acetoacetate shows, which type of isomerism?
A. Chain
B. Optical
C. Metamerism
D. Tautomerism
Answer
597k+ views
Hint: “Isomerism is the phenomenon in which more than one compound has the same chemical formula but different chemical structures”. Remember that all compounds won’t show the property of isomers. Based on the isomerism the name of the molecules are going to change.
Complete step by step answer:
The given compound is Ethyl acetoacetate.
The structure of Ethyl acetoacetate is as follows
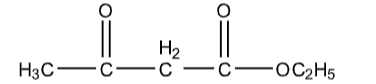
Ethyl acetoacetate contains two functional groups, one is ketone and the other one is ester.
Those two functional groups are separated by a methylene group.
Those methylene hydrogens are highly acidic in nature. They are loosely bound to the molecule.
Those methylene hydrogens are moved towards those functional groups and exhibit an isomerism is called keto-enol tautomerism.
The representation of keto-enol tautomerism in ethyl acetoacetate is as follows.
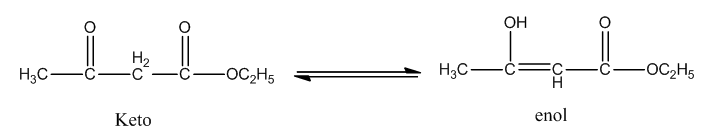
So, ethyl acetoacetate exhibits keto-enol tautomerism.
So, the correct option is D, Tautomerism.
Additional Information:
1) The keto form predominates at equilibrium for most ketones.
2) The basic condition to exhibit keto-enol tautomerism is the presence of acidic hydrogen at the alpha position of the ketone.
3) Acetone also exhibits keto-enol tautomerism.
4) The keto and enol forms always show equilibrium in the solution.
Note: Don’t be confused with the words tautomerism and keto-enol tautomerism. Both are the same.
“Keto–enol tautomerism refers to a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol (an alcohol). The keto and enol forms are said to be tautomers of each other”.
Complete step by step answer:
The given compound is Ethyl acetoacetate.
The structure of Ethyl acetoacetate is as follows

Ethyl acetoacetate contains two functional groups, one is ketone and the other one is ester.
Those two functional groups are separated by a methylene group.
Those methylene hydrogens are highly acidic in nature. They are loosely bound to the molecule.
Those methylene hydrogens are moved towards those functional groups and exhibit an isomerism is called keto-enol tautomerism.
The representation of keto-enol tautomerism in ethyl acetoacetate is as follows.

So, ethyl acetoacetate exhibits keto-enol tautomerism.
So, the correct option is D, Tautomerism.
Additional Information:
1) The keto form predominates at equilibrium for most ketones.
2) The basic condition to exhibit keto-enol tautomerism is the presence of acidic hydrogen at the alpha position of the ketone.
3) Acetone also exhibits keto-enol tautomerism.
4) The keto and enol forms always show equilibrium in the solution.
Note: Don’t be confused with the words tautomerism and keto-enol tautomerism. Both are the same.
“Keto–enol tautomerism refers to a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol (an alcohol). The keto and enol forms are said to be tautomers of each other”.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




