
Ethyl magnesium bromide reacts with methanol to produce:
A. methane
B. methoxyethane
C. ethane
D. propane
Answer
558.9k+ views
Hint:To answer this question we should talk about reagents, reactants, ethyl magnesium bromide, its work. We should also know about acidic hydrogen, nucleophiles. Here, methanol is a reactant and ethyl magnesium bromide is a reagent. Ethyl magnesium bromide is a nucleophilic reagent. It gives ethyl nucleophiles.
Complete answer:
The chemical formula of methanol is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}$. The chemical formula of ethyl magnesium bromide${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}}$.
The reagent ethyl magnesium bromide dissociates as follows:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}_2^ - \,{\text{ + }}\,{\text{M}}{{\text{g}}^ + }{\text{Br}}$
In methanol two types of hydrogens are present. Type-one that are attached to carbon and type-second that are attached to oxygen. The hydrogen attached with oxygen is most acidic and loose as proton, so ethyl nucleophile attacks on hydrogen attached with oxygen in methanol and forms ethane. The negative charge of oxygen is balanced by positively charged magnesium bromide.
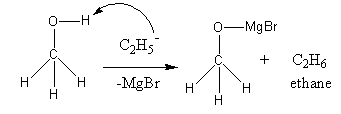
So, the ethyl magnesium bromide reacts with methanol to produce ethane.
Therefore, option (C) ethane is correct.
Note:The electronegativity of oxygen is more than carbon so, the hydrogen attached with oxygen is most acidic. The attacker having a negative charge is known as a nucleophile. The attack having a positive charge is known as an electrophile. An ethyl group is an alkali group. The RMgX is known as alkyl magnesium bromide. The alkyl magnesium bromide is known as the Grignard reagent. The Grignard reagent is used for the preparation of alkyl nucleophiles. As the ethyl nucleophile substitutes the methoxy nucleophile from the methanol so, this is a nucleophilic substitution reaction. The ${\text{C}}{{\text{H}}_{\text{3}}}{\text{O}}$ is known as the methoxy group.
Complete answer:
The chemical formula of methanol is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}$. The chemical formula of ethyl magnesium bromide${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}}$.
The reagent ethyl magnesium bromide dissociates as follows:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}_2^ - \,{\text{ + }}\,{\text{M}}{{\text{g}}^ + }{\text{Br}}$
In methanol two types of hydrogens are present. Type-one that are attached to carbon and type-second that are attached to oxygen. The hydrogen attached with oxygen is most acidic and loose as proton, so ethyl nucleophile attacks on hydrogen attached with oxygen in methanol and forms ethane. The negative charge of oxygen is balanced by positively charged magnesium bromide.

So, the ethyl magnesium bromide reacts with methanol to produce ethane.
Therefore, option (C) ethane is correct.
Note:The electronegativity of oxygen is more than carbon so, the hydrogen attached with oxygen is most acidic. The attacker having a negative charge is known as a nucleophile. The attack having a positive charge is known as an electrophile. An ethyl group is an alkali group. The RMgX is known as alkyl magnesium bromide. The alkyl magnesium bromide is known as the Grignard reagent. The Grignard reagent is used for the preparation of alkyl nucleophiles. As the ethyl nucleophile substitutes the methoxy nucleophile from the methanol so, this is a nucleophilic substitution reaction. The ${\text{C}}{{\text{H}}_{\text{3}}}{\text{O}}$ is known as the methoxy group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




