
Ethylene forms ethylene chlorohydrin by the action of :
(A) dry HCl gas
(B) dry chlorine gas
(C) solution of chlorine gas in water
(D) dilute hydrochloric acid
Answer
591.9k+ views
Hint: 2-chloro ethanol is a chemical named as ethylene chlorohydrin. The most important property of ethylene chlorohydrin is its capacity to give up ${{H}^{+}}$ and $C{{l}^{-}}$ , as a reaction with sodium hydroxide.
Complete answer:
Let us check while ethylene reacts with all substances given in the question.
(A) ethylene reacts with dry HCl gas, this is a type of electrophilic addition reaction.
\[HCl\to {{H}^{+}}+C{{l}^{-}}\]
${{H}^{+}}$ is an electrophile, which added to ethylene forms ethyl cation,
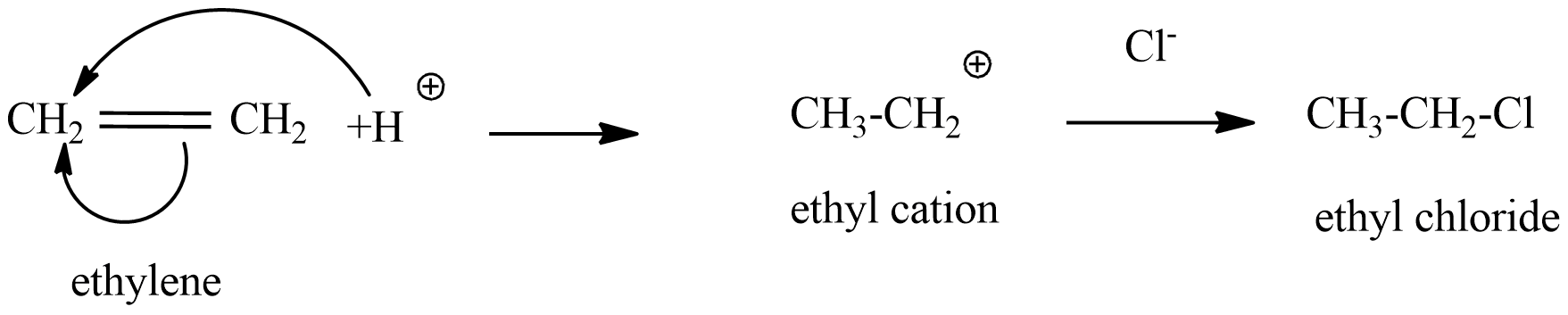
From the above reaction, when ethylene reacts with dry HCl gas, ethyl chloride will form. Hence in this reaction ethylene chlorohydrin does not exist in the product.
(B) ethylene reacts with dry chlorine gas, which forms ethylene dichloride. The resulting chemical reaction is as follows,
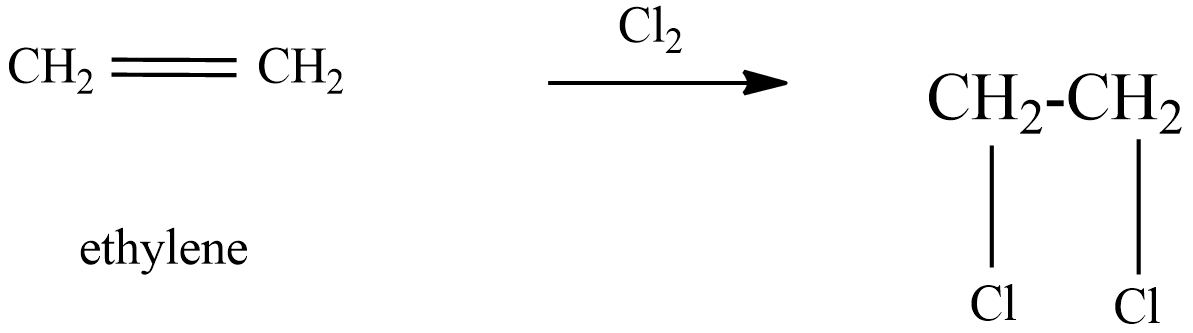
In this reaction also ethylene chlorohydrins are not formed in the product. Hence there is no formation of ethylene chlorohydrins from ethylene reacts with dry chlorine gas.
(C) ethylene reacts with a solution of chlorine gas in water.
In this reaction, firstly when chlorine gas added in water forms hypochlorous acid (HOCl)
\[C{{l}_{2}}+{{H}_{2}}O\to HCl+HOCl\]
This Hypochlorous acid reacts with ethylene and forms 2-chloro ethanol, which is also chemically known as ethylene chlorohydrin.
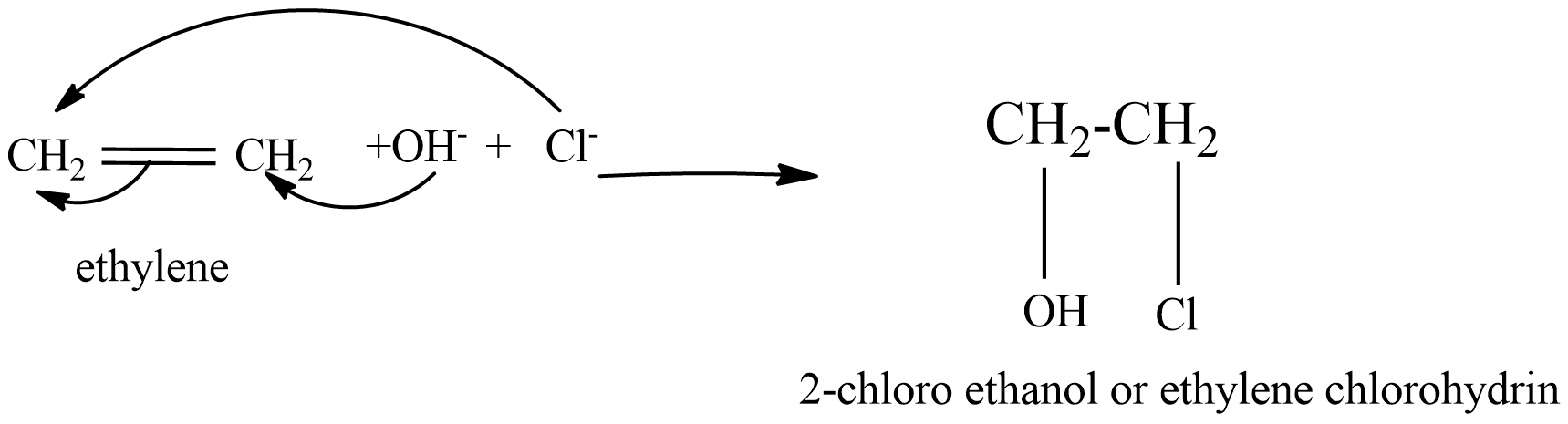
(D) ethylene reacts with dilute hydrochloric acid, the addition reaction takes place form chloroethane.
From the above observation, ethylene forms ethylene chlorohydrins by the action of a solution of chlorine gas in water.
So, the correct answer is “Option C”.
Note: Ethylene chlorohydrins readily dissolve in many organic substances like cellulose acetate and ethylcellulose. But it is used as a solvent and has extremely limited access due to its toxicity. The maximum permissible concentration as vapor in the air is $0.5mg/{{m}^{3}}$ .
Complete answer:
Let us check while ethylene reacts with all substances given in the question.
(A) ethylene reacts with dry HCl gas, this is a type of electrophilic addition reaction.
\[HCl\to {{H}^{+}}+C{{l}^{-}}\]
${{H}^{+}}$ is an electrophile, which added to ethylene forms ethyl cation,
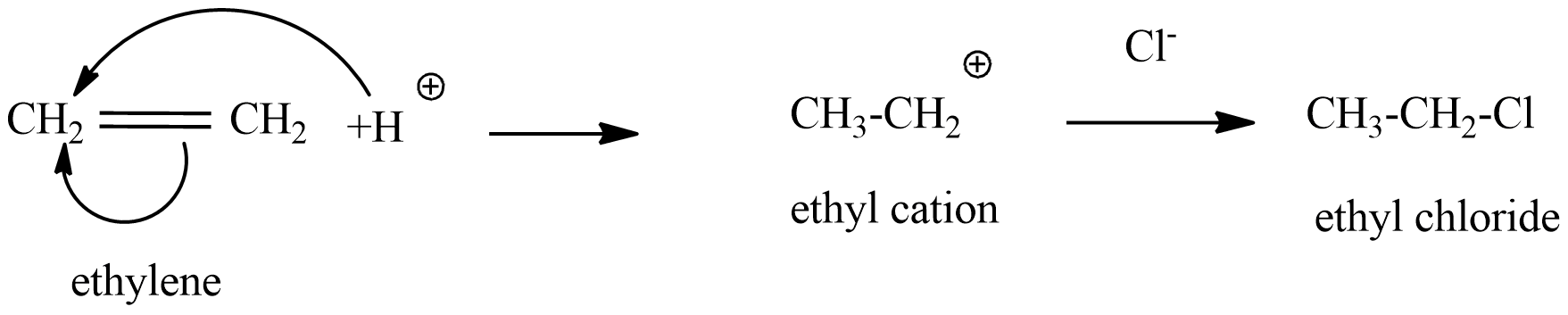
From the above reaction, when ethylene reacts with dry HCl gas, ethyl chloride will form. Hence in this reaction ethylene chlorohydrin does not exist in the product.
(B) ethylene reacts with dry chlorine gas, which forms ethylene dichloride. The resulting chemical reaction is as follows,

In this reaction also ethylene chlorohydrins are not formed in the product. Hence there is no formation of ethylene chlorohydrins from ethylene reacts with dry chlorine gas.
(C) ethylene reacts with a solution of chlorine gas in water.
In this reaction, firstly when chlorine gas added in water forms hypochlorous acid (HOCl)
\[C{{l}_{2}}+{{H}_{2}}O\to HCl+HOCl\]
This Hypochlorous acid reacts with ethylene and forms 2-chloro ethanol, which is also chemically known as ethylene chlorohydrin.

(D) ethylene reacts with dilute hydrochloric acid, the addition reaction takes place form chloroethane.
From the above observation, ethylene forms ethylene chlorohydrins by the action of a solution of chlorine gas in water.
So, the correct answer is “Option C”.
Note: Ethylene chlorohydrins readily dissolve in many organic substances like cellulose acetate and ethylcellulose. But it is used as a solvent and has extremely limited access due to its toxicity. The maximum permissible concentration as vapor in the air is $0.5mg/{{m}^{3}}$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




