
Ethylenediamine is an example of:
a.) Monodentate ligand
b.) Bidentate ligand
c.) Tridentate ligand
d.) Polydentate ligand
Answer
567k+ views
Hint: The term ligand comes from a Latin word ligare. The word ligare means bind. Ligands are the ions or neutral molecules which bond to a central metal atom or an ion.
Complete Solution :
- Ligands act as a Lewis base which means that ligands donate electron pairs and the central atom acts as a Lewis acid which means that the central metal atom accepts the pair of electrons. Ligands can be anion, cation or neutral molecules which means that they can either be negatively charged or positively charged or neutral.
- A ligand and transition metal combinedly form coordination compounds. The coordination compounds have different geometries and the geometry of a compound depends on the coordination number and the hybridization.
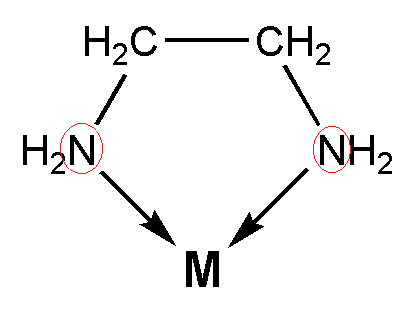
- Ethylenediamine is an example of bidentate ligand. This means that ethylene diamine has two donor atoms. It means that it can bind with the central metal ion with two nitrogen atoms. The bidentate ligands are also known as chelating ligands. The complexes which contain the chelating ligands are called chelates. So, the correct answer is “Option B”.
Note: Non-chelating ligands are the ligands that bond to just one site. For example: chloride, cyanide and water. Some examples of naturally occurring chelating agents are citric, malic and lactic acid. The monodentate ligands bind to the central metal ion through only one donor atom. This is why they are also known as one- tooth.
Complete Solution :
- Ligands act as a Lewis base which means that ligands donate electron pairs and the central atom acts as a Lewis acid which means that the central metal atom accepts the pair of electrons. Ligands can be anion, cation or neutral molecules which means that they can either be negatively charged or positively charged or neutral.
- A ligand and transition metal combinedly form coordination compounds. The coordination compounds have different geometries and the geometry of a compound depends on the coordination number and the hybridization.

- Ethylenediamine is an example of bidentate ligand. This means that ethylene diamine has two donor atoms. It means that it can bind with the central metal ion with two nitrogen atoms. The bidentate ligands are also known as chelating ligands. The complexes which contain the chelating ligands are called chelates. So, the correct answer is “Option B”.
Note: Non-chelating ligands are the ligands that bond to just one site. For example: chloride, cyanide and water. Some examples of naturally occurring chelating agents are citric, malic and lactic acid. The monodentate ligands bind to the central metal ion through only one donor atom. This is why they are also known as one- tooth.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




