
Except for isopropyl alcohol all iso and neo alcohols are _________ alcohol.
A. Primary
B. Secondary
C. Tertiary
D. Quaternary
Answer
607.8k+ views
Hint- In order to solve this problem first we will see the structure of isopropyl alcohol and some other iso and neo alcohols. On the basis of their structure and comparison we will select the correct option amongst the given options.
Complete answer:
The structure of isopropyl alcohol is:
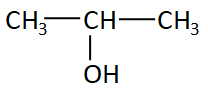
Here the alcohol group is attached with the second carbon in the chain so this structure is secondary or ${2^0}$ structure.
Now let us see some other iso and neo alcohol
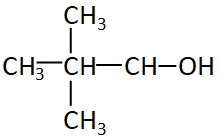
The figure given above is neopentyl alcohol, even if the alcohol group is attached to any of the carbon of neopentyl it will always be the first carbon or the edge carbon. So the structure will always be ${1^0}$ or primary structure.
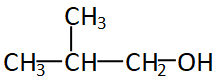
The figure given above is iso-butyl alcohol, even if the alcohol group is attached to any of the carbon of iso-but it will always be the first carbon or the edge carbon. So the structure will always be ${1^0}$ or primary structure.
Similar will be the results for other iso and neo alcohol as well.
Hence, on the basis of these results we conclude that:
Except for isopropyl alcohol all iso and neo alcohols are primary alcohol.
So, option A is the correct option.
Note- The prefix "iso" is used when all carbons except one form a continuous chain. The "neo" suffix is used where all but two carbons form a continuous line, and those two carbons are part of a tert-butyl group of terminals. A primary alcohol is an alcohol in which a single carbon atom is bound to the hydroxyl groups. It can also be defined as a molecule containing a $ - C{H_2}OH$ group. In contrast, a secondary alcohol has a formula $ - CHROH$ and a tertiary alcohol has a formula $ - C{R_2}OH$ , where “R” indicates a carbon-containing group.
Complete answer:
The structure of isopropyl alcohol is:

Here the alcohol group is attached with the second carbon in the chain so this structure is secondary or ${2^0}$ structure.
Now let us see some other iso and neo alcohol

The figure given above is neopentyl alcohol, even if the alcohol group is attached to any of the carbon of neopentyl it will always be the first carbon or the edge carbon. So the structure will always be ${1^0}$ or primary structure.
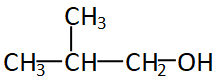
The figure given above is iso-butyl alcohol, even if the alcohol group is attached to any of the carbon of iso-but it will always be the first carbon or the edge carbon. So the structure will always be ${1^0}$ or primary structure.
Similar will be the results for other iso and neo alcohol as well.
Hence, on the basis of these results we conclude that:
Except for isopropyl alcohol all iso and neo alcohols are primary alcohol.
So, option A is the correct option.
Note- The prefix "iso" is used when all carbons except one form a continuous chain. The "neo" suffix is used where all but two carbons form a continuous line, and those two carbons are part of a tert-butyl group of terminals. A primary alcohol is an alcohol in which a single carbon atom is bound to the hydroxyl groups. It can also be defined as a molecule containing a $ - C{H_2}OH$ group. In contrast, a secondary alcohol has a formula $ - CHROH$ and a tertiary alcohol has a formula $ - C{R_2}OH$ , where “R” indicates a carbon-containing group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




