
Explain aldol condensation by taking acetaldehyde.
Answer
513.3k+ views
Hint: Aldol condensation is the reaction when two aldehydes that are same or different react together in the presence of dilute alkali to form a product intermediate that has an aldehyde and alcohol, called as aldol. This aldol on dehydration gives alpha, beta – unsaturated carbonyl compounds. This is termed as aldol reaction.
Complete answer:
Aldol means an alcohol and an aldehyde group on the same compound. Aldol condensation is the condensation reaction between two aldehydes or ketones that may be same or different in the presence of dilute alkali (NaOH), to form a compound called as aldol that has both aldehyde and alcohol.
For aldol condensation to take place, alpha – hydrogen is a must that is the hydrogen attached with the carbon adjacent to the beta – carbon. This is because it is acidic and attached with a very strong electron withdrawing group that results in the anion that can be stabilized by resonance.
The aldol condensation takes place as the two aldehydes, for example of acetaldehyde, condense together to form an aldol called 3 – hydroxybutanal as follows:
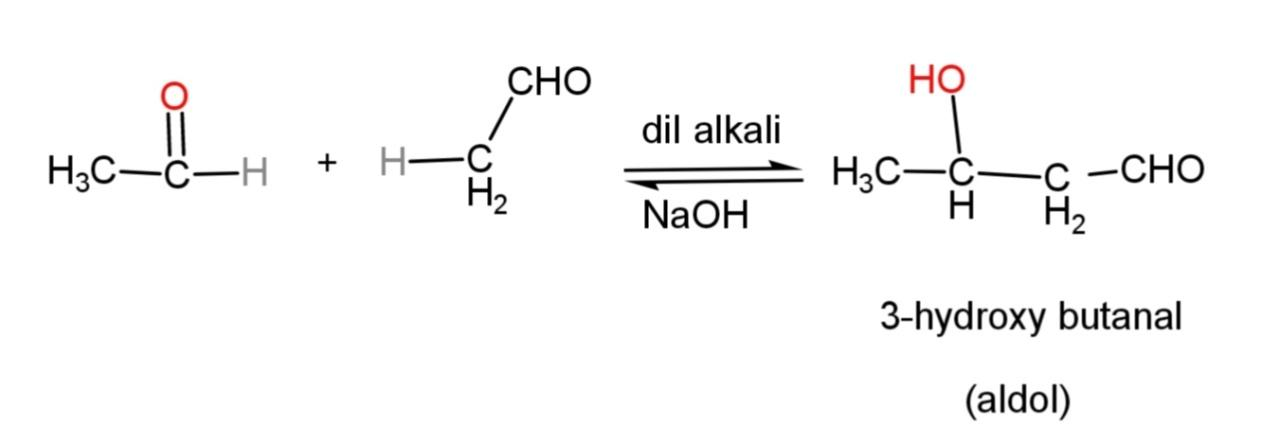
This aldol when subjected to dehydration by heating, it loses water molecule to form $\alpha ,\beta $- unsaturated compound as follows:
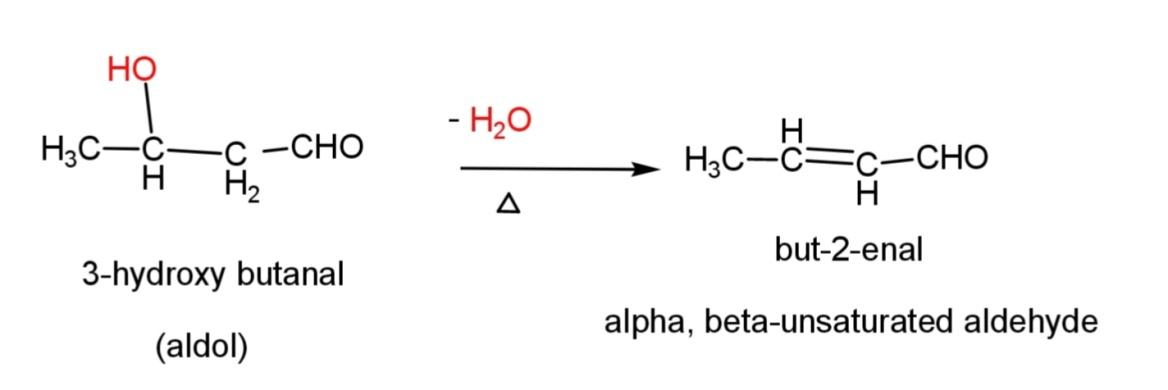
Here, but – 2 – enal is formed as the compound.
Hence, aldol condensation is explained by the above reactions of acetaldehyde.
Note:
The reaction can also be carried out with ketones and $\alpha ,\beta $- unsaturated ketones are formed as a result of ketol condensation that consist of reacting two ketones same or different in presence of a dilute alkali, which forms a ketol intermediate having an alcohol and a ketone group that on heating produces$\alpha ,\beta $- unsaturated ketone. When different aldehyde or ketones react then it is called cross aldol or ketol condensation, then 4 products are formed.
Complete answer:
Aldol means an alcohol and an aldehyde group on the same compound. Aldol condensation is the condensation reaction between two aldehydes or ketones that may be same or different in the presence of dilute alkali (NaOH), to form a compound called as aldol that has both aldehyde and alcohol.
For aldol condensation to take place, alpha – hydrogen is a must that is the hydrogen attached with the carbon adjacent to the beta – carbon. This is because it is acidic and attached with a very strong electron withdrawing group that results in the anion that can be stabilized by resonance.
The aldol condensation takes place as the two aldehydes, for example of acetaldehyde, condense together to form an aldol called 3 – hydroxybutanal as follows:

This aldol when subjected to dehydration by heating, it loses water molecule to form $\alpha ,\beta $- unsaturated compound as follows:

Here, but – 2 – enal is formed as the compound.
Hence, aldol condensation is explained by the above reactions of acetaldehyde.
Note:
The reaction can also be carried out with ketones and $\alpha ,\beta $- unsaturated ketones are formed as a result of ketol condensation that consist of reacting two ketones same or different in presence of a dilute alkali, which forms a ketol intermediate having an alcohol and a ketone group that on heating produces$\alpha ,\beta $- unsaturated ketone. When different aldehyde or ketones react then it is called cross aldol or ketol condensation, then 4 products are formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




