
Explain Davisson and Germer experiment to verify the wave nature of electrons.
Answer
515.4k+ views
Hint: Subatomic particles with an elementary charge of -1 are known as electrons. The charge carried by an electron is the same as the charge held by a proton (but has an opposite sign). As a result, electrically neutral atoms/molecules would have the same number of protons and electrons.
Complete step-by-step answer:
Scientists' initial atomic models could only describe the particle nature of electrons, but not the properties relevant to their wave nature. In 1927, C.J. Davisson and L.H. Germer conducted an experiment known as the Davisson Germer experiment to demonstrate the wave nature of electrons by electron diffraction. We'll read about the experiment's findings and results in this post.
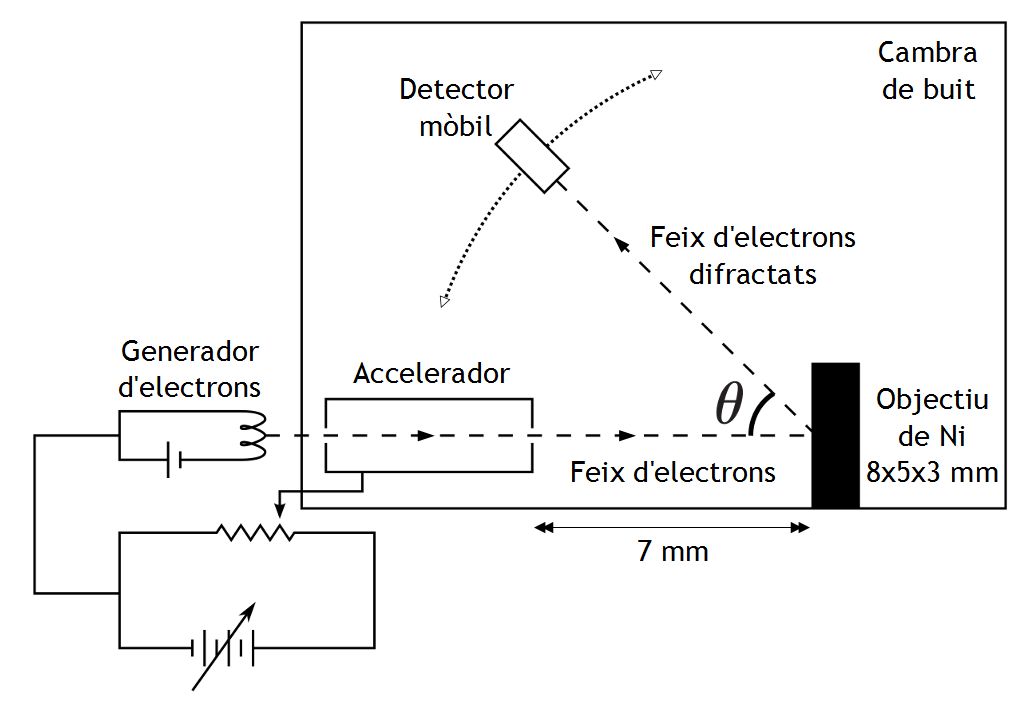
The Davisson Germer experiment's experimental setup is described below:
A low voltage power supply was used to heat an electron gun with a tungsten filament F coated with barium oxide.
The electron gun releases electrons that are accelerated to a certain velocity using an appropriate potential gap from a high voltage power source.
These released electrons were made to travel through a cylinder perforated with fine holes along its axis, resulting in a fine collimated beam.
The cylinder's beam is made to land on the top of a nickel crystal once more. The electrons scatter in different ways as a result of this.
The strength of the beam of electrons emitted is determined by the electron detector, which is then attached to a sensitive galvanometer (to record the current) and moved on a circular scale.
The amplitude of the dispersed electron beam is determined with various values of angle of scattering by rotating the detector on the circular scale at different points, increasing the$\theta $ (angle between the incident and scattered electron beams).
 Inferences:
Inferences:
The following observations can be drawn from this experiment:
By changing the angle of scattering\[\theta \], we were able to achieve variations in the amplitude (I) of the dispersed electrons.
The accelerated voltage was changed from 44V to 68V by changing the accelerating potential difference.
We might see a clear peak in the strength (I) of the scattered electron with an accelerated voltage of 54V at a scattering angle of\[\theta = {50^o}\].
The positive intrusion of electrons dispersed from various layers of the regularly spaced atoms of the crystals produced this peak.
The wavelength of matter waves was measured using electron diffraction and found to be 0.165 nm.
Note: The Davisson and Germer experiment shows that electrons are replaced by wave particles. A diffraction pattern was created as these electrons came together. Finally, the dual essence of matter is generated as the final product.
The electron diffraction chapter of the Davisson Germer experiment is complicated in terms of equation and application. A pupil must have a thorough knowledge of the fundamental topics in order to achieve high grades. This difficult subject necessitates students relying on guidebooks and research materials that only include lessons rather than a thorough description.
Complete step-by-step answer:
Scientists' initial atomic models could only describe the particle nature of electrons, but not the properties relevant to their wave nature. In 1927, C.J. Davisson and L.H. Germer conducted an experiment known as the Davisson Germer experiment to demonstrate the wave nature of electrons by electron diffraction. We'll read about the experiment's findings and results in this post.
The Davisson Germer experiment's experimental setup is described below:
A low voltage power supply was used to heat an electron gun with a tungsten filament F coated with barium oxide.
The electron gun releases electrons that are accelerated to a certain velocity using an appropriate potential gap from a high voltage power source.
These released electrons were made to travel through a cylinder perforated with fine holes along its axis, resulting in a fine collimated beam.
The cylinder's beam is made to land on the top of a nickel crystal once more. The electrons scatter in different ways as a result of this.
The strength of the beam of electrons emitted is determined by the electron detector, which is then attached to a sensitive galvanometer (to record the current) and moved on a circular scale.
The amplitude of the dispersed electron beam is determined with various values of angle of scattering by rotating the detector on the circular scale at different points, increasing the$\theta $ (angle between the incident and scattered electron beams).

The following observations can be drawn from this experiment:
By changing the angle of scattering\[\theta \], we were able to achieve variations in the amplitude (I) of the dispersed electrons.
The accelerated voltage was changed from 44V to 68V by changing the accelerating potential difference.
We might see a clear peak in the strength (I) of the scattered electron with an accelerated voltage of 54V at a scattering angle of\[\theta = {50^o}\].
The positive intrusion of electrons dispersed from various layers of the regularly spaced atoms of the crystals produced this peak.
The wavelength of matter waves was measured using electron diffraction and found to be 0.165 nm.
Note: The Davisson and Germer experiment shows that electrons are replaced by wave particles. A diffraction pattern was created as these electrons came together. Finally, the dual essence of matter is generated as the final product.
The electron diffraction chapter of the Davisson Germer experiment is complicated in terms of equation and application. A pupil must have a thorough knowledge of the fundamental topics in order to achieve high grades. This difficult subject necessitates students relying on guidebooks and research materials that only include lessons rather than a thorough description.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




