
Explain the following with one example:
(a) Williamson’s synthesis
(b) Kolbe’s reaction
(c) Hell-volhard-zelinsky $\left( {HVZ} \right)$ reaction.
(d) Aldol reaction.
Answer
582.3k+ views
Hint: In organic reactions covalent bonds break and some new covalent bonds are formed . This making and breaking of covalent bonds usually occur in a number of steps before they are finally turned into products ; the sequential description of all the steps of the transformation into product is called the mechanism of a reaction.
Complete step by step answer:
(a) Williamson’s synthesis: It is the method used for the production of ethers . It occurs by an $S{N^2}$ reaction in which a metal alkoxide displaces a halide ion from an alkyl halide. The alkoxide ion is prepared by the reaction of an alcohol with a strong base such as sodium Hydride
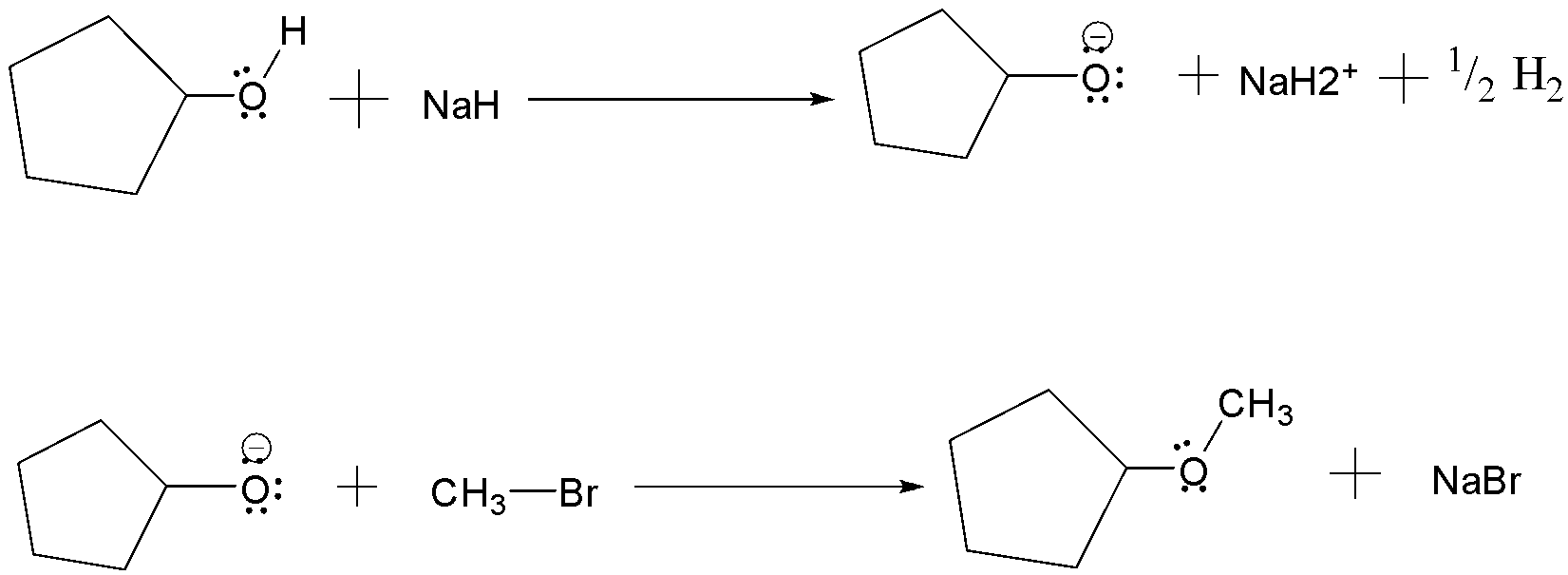
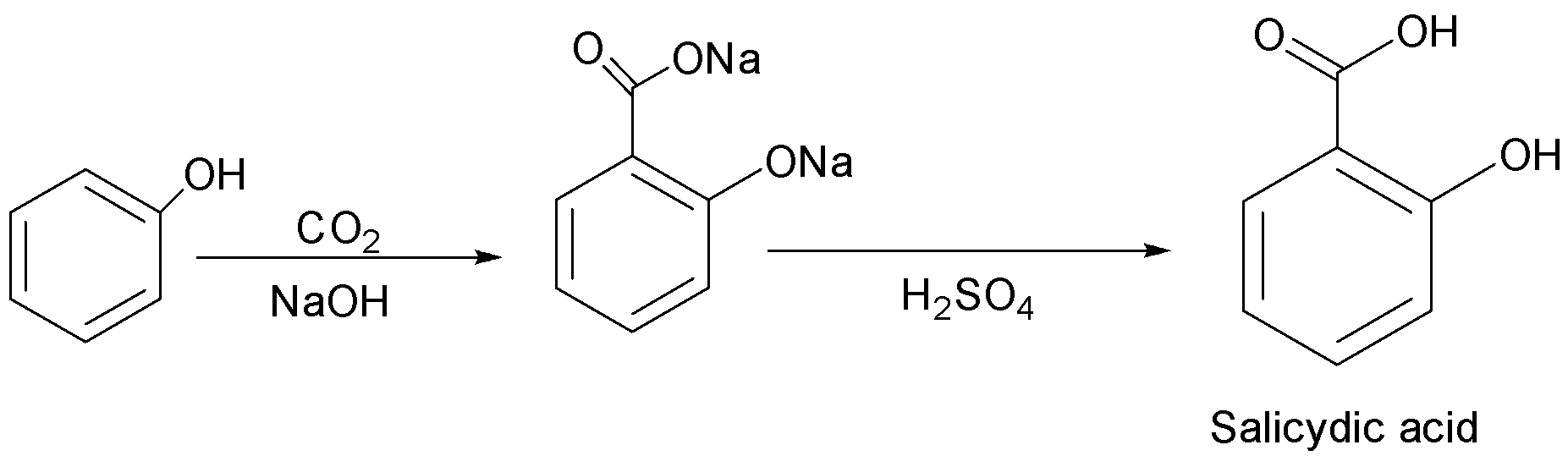
(b) Kolbe’s reaction: It is a chemical reaction that begins by first heating sodium phenoxide(the sodium salt of phenol) with carbon dioxide under pressure $\left( {100\;{\text{atm}},{{125}^ \circ }C} \right)$ , then the product is treated with sulphuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.
(c) Hell-volhard-zelinsky $\left( {HVZ} \right)$ reaction: When carboxylic acid reacts with chlorine or bromine using red phosphorus then halo atom is substituted at the Alpha carbon and the product obtained is mono $\alpha $ -halogenated acids. This is known as the $HVZ$ reaction.
$C{H_3}C{H_2}COOH + B{r_2}\xrightarrow{{{\text{Red}}\;{\text{P}}}}C{H_3}CHBrCOOH + HBr$
(d) Aldol reaction: The word aldol means product having both aldehyde and alcoholic group
Aldehyde having $\alpha $ -hydrogen(s) undergoes self condensation on warning with dilute or mild base to give $\beta - $ hydroxy aldehyde, called aldol (aldehyde $ + $ alcohol). This reaction is known as aldol condensation.
Note:The mechanism of a reaction is a very important part because initially we think the reactants will produce something but when we look at the mechanism we find out that actually the product is something else .
Complete step by step answer:
(a) Williamson’s synthesis: It is the method used for the production of ethers . It occurs by an $S{N^2}$ reaction in which a metal alkoxide displaces a halide ion from an alkyl halide. The alkoxide ion is prepared by the reaction of an alcohol with a strong base such as sodium Hydride

(b) Kolbe’s reaction: It is a chemical reaction that begins by first heating sodium phenoxide(the sodium salt of phenol) with carbon dioxide under pressure $\left( {100\;{\text{atm}},{{125}^ \circ }C} \right)$ , then the product is treated with sulphuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.

(c) Hell-volhard-zelinsky $\left( {HVZ} \right)$ reaction: When carboxylic acid reacts with chlorine or bromine using red phosphorus then halo atom is substituted at the Alpha carbon and the product obtained is mono $\alpha $ -halogenated acids. This is known as the $HVZ$ reaction.
$C{H_3}C{H_2}COOH + B{r_2}\xrightarrow{{{\text{Red}}\;{\text{P}}}}C{H_3}CHBrCOOH + HBr$
(d) Aldol reaction: The word aldol means product having both aldehyde and alcoholic group
Aldehyde having $\alpha $ -hydrogen(s) undergoes self condensation on warning with dilute or mild base to give $\beta - $ hydroxy aldehyde, called aldol (aldehyde $ + $ alcohol). This reaction is known as aldol condensation.
Note:The mechanism of a reaction is a very important part because initially we think the reactants will produce something but when we look at the mechanism we find out that actually the product is something else .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




