
Explain the formation of ammonia molecule
Answer
573.6k+ views
Hint: The chemical formula of ammonia is $N{{H}_{3}}$. We need to know about the structure in detail to know about the formation. The maximum oxidation state of nitrogen is +5, so the ammonia will form by some reduction process of the nitrogen.
Complete answer:
Ammonia, a colorless gas with a definite odor, may be a building-block chemical and a key component within the manufacture of the many products people use each day. Ammonia can be found naturally throughout the environment within the air, soil and water and in plants and animals, including humans. The physical body makes ammonia when the body breaks down foods containing protein into amino acids and ammonia, then converting the ammonia into urea.
Ammonium hydroxide – commonly referred to as household ammonia – is an ingredient in many everyday household cleaning products. Ammonia could be a basic building block for nitrate fertilizer, which releases nitrogen, an important nutrient for growing plants, including farm crops and lawns. About 90 percent of ammonia produced is employed in fertilizer, to assist sustain food production for billions of individuals round the world. Ammonia has other important uses; as an example in household cleaning products and in manufacturing other products. Ammonia occurs naturally and is found throughout the environment in soil, air, and water. Ammonia is also renewed naturally as a part of the biological process that already occurs as plants fertilize. As a result of this activity, ammonia doesn't last long within the environment, and it also doesn't bioaccumulate. Now let's come to the solution:
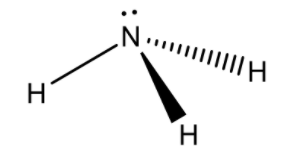
Ammonia consists of 1 nitrogen atom and 3 hydrogen atoms. The 3 hydrogen atoms share their valence electron with nitrogen to form 3 covalent bonds and to attain a stable electronic configuration. That is how ammonia is formed.
Note: Ammonia encompasses a very distinct, pungent odor, described as just like sweat or cat urine. Strong, briny cheeses like brie also can smell like ammonia. Ammonia is formed using the Haber’s process that is:
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\to 2N{{H}_{3}}(g)\]
Complete answer:
Ammonia, a colorless gas with a definite odor, may be a building-block chemical and a key component within the manufacture of the many products people use each day. Ammonia can be found naturally throughout the environment within the air, soil and water and in plants and animals, including humans. The physical body makes ammonia when the body breaks down foods containing protein into amino acids and ammonia, then converting the ammonia into urea.
Ammonium hydroxide – commonly referred to as household ammonia – is an ingredient in many everyday household cleaning products. Ammonia could be a basic building block for nitrate fertilizer, which releases nitrogen, an important nutrient for growing plants, including farm crops and lawns. About 90 percent of ammonia produced is employed in fertilizer, to assist sustain food production for billions of individuals round the world. Ammonia has other important uses; as an example in household cleaning products and in manufacturing other products. Ammonia occurs naturally and is found throughout the environment in soil, air, and water. Ammonia is also renewed naturally as a part of the biological process that already occurs as plants fertilize. As a result of this activity, ammonia doesn't last long within the environment, and it also doesn't bioaccumulate. Now let's come to the solution:

Ammonia consists of 1 nitrogen atom and 3 hydrogen atoms. The 3 hydrogen atoms share their valence electron with nitrogen to form 3 covalent bonds and to attain a stable electronic configuration. That is how ammonia is formed.
Note: Ammonia encompasses a very distinct, pungent odor, described as just like sweat or cat urine. Strong, briny cheeses like brie also can smell like ammonia. Ammonia is formed using the Haber’s process that is:
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\to 2N{{H}_{3}}(g)\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




