
Explain the hybridization in the ethane molecule. Draw the structure to demonstrate chemical bonding in it.
Answer
598.8k+ views
Hint: Hybridization of a molecule can also be calculated by a formula:
Hybridization = \[\dfrac{1}{2}(V+M-C+A)\]
After getting the numerical value,
2 = \[sp\]
3 = \[s{{p}^{2}}\]
4 = \[s{{p}^{3}}\]
5 = \[s{{p}^{3}}d\]
6 = \[s{{p}^{3}}{{d}^{2}}\]
Complete step by step solution:
First let’s draw the structure of ethane molecule.
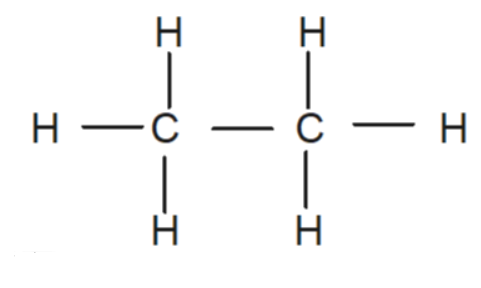
From the structure we can see each carbon forms four bonds, three bonds with a Hydrogen atom and one with the adjacent carbon atom. To find the hybridisation of the molecule, first we will isolate both of the Carbon atoms and write down their electron configuration, which is 1s2 2s2 2p2. From the electronic configuration we can see that only two unpaired electrons are available in orbital but we know that carbon can form four bonds, making it obvious that hybridization is required to make the four unpaired electrons available for this bonding. As a result, four sp3 hybrid orbitals are formed on each carbon.
Now, both of the carbons will have four bonds arranged in tetrahedral geometry. The carbon-carbon sigma bond is formed by overlapping of the sp3 hybrid orbital of the one carbon with the other carbon. which also has three Hydrogens bonded to it in the similar manner, while the six carbon-hydrogen sigma bonds are formed from overlaps between the sp3 orbitals on the two carbons and the 1s orbitals of six hydrogen atoms. And we get a molecule with a total of seven sigma bonds and eventually the ethane molecule.
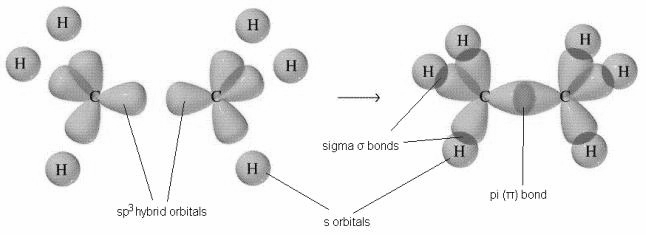
Note: Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. In this case of ethane molecules, the two methyl (CH3) groups are free to rotate.
Hybridization = \[\dfrac{1}{2}(V+M-C+A)\]
where, V = valence electrons,
M = monovalent atom linked to central atom,
C = charge on cation,
A = charge on anion.
After getting the numerical value,
2 = \[sp\]
3 = \[s{{p}^{2}}\]
4 = \[s{{p}^{3}}\]
5 = \[s{{p}^{3}}d\]
6 = \[s{{p}^{3}}{{d}^{2}}\]
Complete step by step solution:
First let’s draw the structure of ethane molecule.

From the structure we can see each carbon forms four bonds, three bonds with a Hydrogen atom and one with the adjacent carbon atom. To find the hybridisation of the molecule, first we will isolate both of the Carbon atoms and write down their electron configuration, which is 1s2 2s2 2p2. From the electronic configuration we can see that only two unpaired electrons are available in orbital but we know that carbon can form four bonds, making it obvious that hybridization is required to make the four unpaired electrons available for this bonding. As a result, four sp3 hybrid orbitals are formed on each carbon.
Now, both of the carbons will have four bonds arranged in tetrahedral geometry. The carbon-carbon sigma bond is formed by overlapping of the sp3 hybrid orbital of the one carbon with the other carbon. which also has three Hydrogens bonded to it in the similar manner, while the six carbon-hydrogen sigma bonds are formed from overlaps between the sp3 orbitals on the two carbons and the 1s orbitals of six hydrogen atoms. And we get a molecule with a total of seven sigma bonds and eventually the ethane molecule.

Note: Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. In this case of ethane molecules, the two methyl (CH3) groups are free to rotate.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




