
Explain the molecular orbital structure of benzene.
Answer
541.8k+ views
Hint: : The benzene molecule contains six carbon atoms and each carbon is $s{{p}^{2}}$hybridized, and the $2{{p}_{z}}$ orbitals overlap to give two sets of $\pi $bonds.
Complete step-by-step answer:
Benzene is an organic compound that has the formula of ${{C}_{6}}{{H}_{6}}$. It is an aromatic compound and consists of a single ring structure . The carbon atoms are $s{{p}^{2}}$ hybridised in benzene and all of them lie in the same plane. They have a ${{120}^{0}}$angle orientation. Perpendicular to the plane of the hybrid orbital, there exists an unhybridized p orbital which contains two lobes. There are three $s{{p}^{2}}$hybrid orbitals of each carbon and from these, two of the orbitals overlap interracially, with neighbouring orbitals so that $\sigma -\sigma $carbon bonds are formed.
The leftover third hybrid orbital of each carbon undergoes overlapping with the hydrogen’s half filled 1s orbital and forms ${{\sigma }_{C}}-{{\sigma }_{H}}$ bond. So, in total there are 12 sigma bonds, where 6 of them are carbon-carbon and the other six are carbon-hydrogen. However, in each carbon atom there exists one unhybridized $2{{p}_{z}}$ orbital.
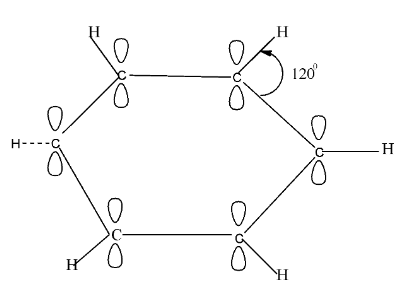
The $2{{p}_{z}}$ orbital which is unhybridized overlaps with the adjacent carbon atom’s $2{{p}_{z}}$ orbital and forms a continuous $\pi $molecular network, and incorporates the six $\pi $ electrons.
Finally, as a result of this, we get the formation of two clouds of electrons shaped in form of rings, one above and one below the plane of atoms.
Note: It is to be noted that the electrons in the benzene ring are delocalised, and this delocalisation provides stability to the benzene molecule. The bond length of carbon attacked to another carbon is 139 pm.
Complete step-by-step answer:
Benzene is an organic compound that has the formula of ${{C}_{6}}{{H}_{6}}$. It is an aromatic compound and consists of a single ring structure . The carbon atoms are $s{{p}^{2}}$ hybridised in benzene and all of them lie in the same plane. They have a ${{120}^{0}}$angle orientation. Perpendicular to the plane of the hybrid orbital, there exists an unhybridized p orbital which contains two lobes. There are three $s{{p}^{2}}$hybrid orbitals of each carbon and from these, two of the orbitals overlap interracially, with neighbouring orbitals so that $\sigma -\sigma $carbon bonds are formed.
The leftover third hybrid orbital of each carbon undergoes overlapping with the hydrogen’s half filled 1s orbital and forms ${{\sigma }_{C}}-{{\sigma }_{H}}$ bond. So, in total there are 12 sigma bonds, where 6 of them are carbon-carbon and the other six are carbon-hydrogen. However, in each carbon atom there exists one unhybridized $2{{p}_{z}}$ orbital.

The $2{{p}_{z}}$ orbital which is unhybridized overlaps with the adjacent carbon atom’s $2{{p}_{z}}$ orbital and forms a continuous $\pi $molecular network, and incorporates the six $\pi $ electrons.
Finally, as a result of this, we get the formation of two clouds of electrons shaped in form of rings, one above and one below the plane of atoms.
Note: It is to be noted that the electrons in the benzene ring are delocalised, and this delocalisation provides stability to the benzene molecule. The bond length of carbon attacked to another carbon is 139 pm.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




