
Explain the preparation of Buna-N with the equation.
Answer
593.1k+ views
Hint: Buna-N is an example of a synthetic rubber. It is prepared by the copolymerization of 1,3-Butadiene and acrylonitrile. Peroxide is used as a catalyst.
Complete step by step answer:
-Buna-N is an example of a synthetic rubber.
-Synthetic rubber may be defined as any vulcanized rubber-like polymer which is capable of getting stretched to twice its length. Synthetic rubbers are either homopolymers of 1,3-butadiene or its derivatives or are copolymers of 1.3-butadiene or its derivatives with another unsaturated monomer. Buna-N is a copolymer rubber.
-Copolymerization is a technique in which two or more different compounds are allowed to polymerize. The product formed after copolymerization is called copolymers. A copolymer can be made not only by chain-growth polymerization but also by step-growth polymerization. It contains a large number of units of each monomer used in the same polymeric chain.
-1,3-Butadiene and acrylonitrile are copolymerized to form Buna-N. This copolymerization takes place in the presence of peroxide as a catalyst.
n number of 1,3-butadiene and n number of acrylonitrile reacts with each other. The double bonds shift to form a single chain. We get n number of Buna-N compounds.
The reaction of formation of Buna-N is given below:
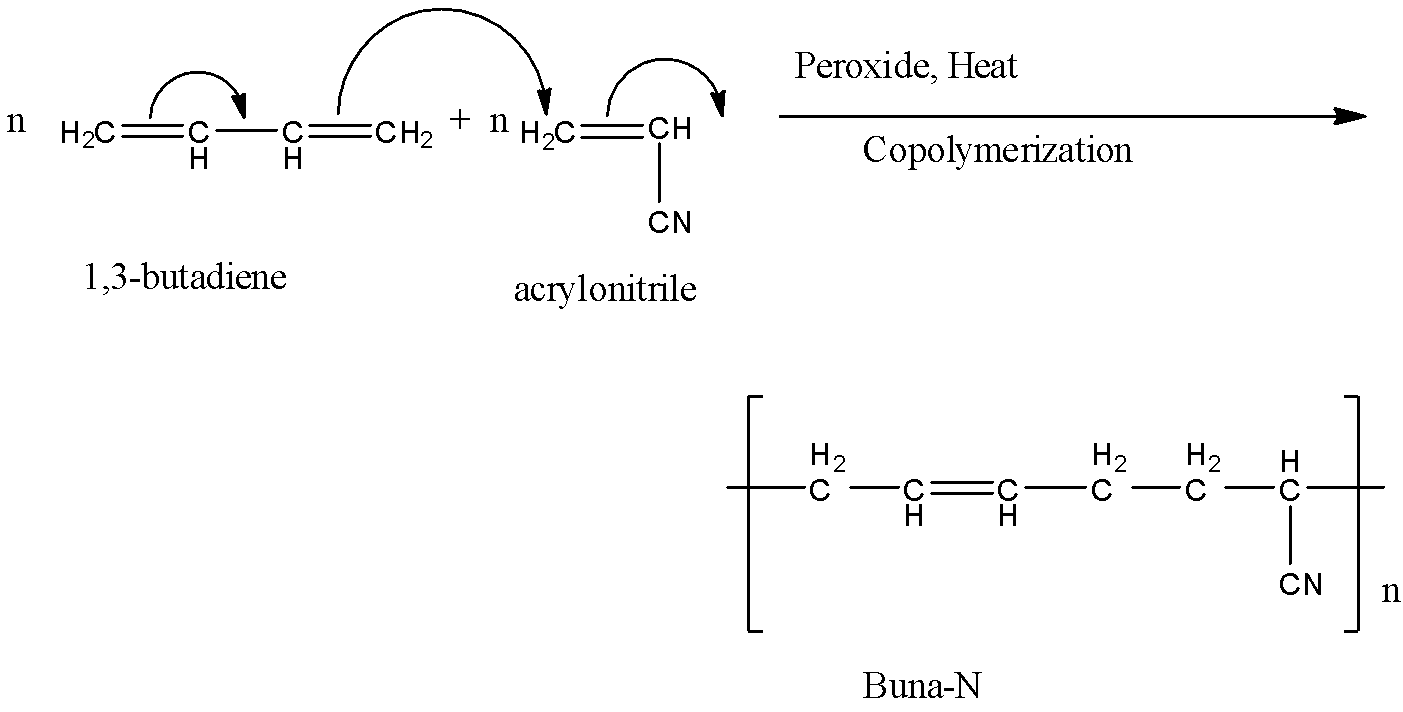
The letter ‘N’ in Buna-N stands for acrylonitrile.
-It doesn’t get affected with the action of petrol, lubrication oil, and organic solvents. It is, therefore, used in making oil seals, hoses, and tank linings, etc.
Note:
Buna-S is also a copolymer which is prepared by the same method. It is prepared by the copolymerization between 1,3-butadiene and styrene. The letter ‘S’ in Buna-S stands for styrene.
Complete step by step answer:
-Buna-N is an example of a synthetic rubber.
-Synthetic rubber may be defined as any vulcanized rubber-like polymer which is capable of getting stretched to twice its length. Synthetic rubbers are either homopolymers of 1,3-butadiene or its derivatives or are copolymers of 1.3-butadiene or its derivatives with another unsaturated monomer. Buna-N is a copolymer rubber.
-Copolymerization is a technique in which two or more different compounds are allowed to polymerize. The product formed after copolymerization is called copolymers. A copolymer can be made not only by chain-growth polymerization but also by step-growth polymerization. It contains a large number of units of each monomer used in the same polymeric chain.
-1,3-Butadiene and acrylonitrile are copolymerized to form Buna-N. This copolymerization takes place in the presence of peroxide as a catalyst.
n number of 1,3-butadiene and n number of acrylonitrile reacts with each other. The double bonds shift to form a single chain. We get n number of Buna-N compounds.
The reaction of formation of Buna-N is given below:

The letter ‘N’ in Buna-N stands for acrylonitrile.
-It doesn’t get affected with the action of petrol, lubrication oil, and organic solvents. It is, therefore, used in making oil seals, hoses, and tank linings, etc.
Note:
Buna-S is also a copolymer which is prepared by the same method. It is prepared by the copolymerization between 1,3-butadiene and styrene. The letter ‘S’ in Buna-S stands for styrene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




