
Explain why evaporation is accompanied by cooling.
Answer
603.3k+ views
Hint: As we know, the evaporation is the change of state from liquid to gas by the liquid at the surface. Vaporization is the general term used for the conversion from the liquid state to solid-state. Atoms and molecules in different states have different energies. In the gaseous state, these atoms are always at random motion and therefore require high energy. While in the solid-state they are tightly packed and require very less energy.
Complete step by step answer:
When atoms and molecules are in the liquid state, there is some interatomic attraction between the nearby molecules. To get transferred to the gaseous state without any extra heat supplied to the surface of the liquid, the liquid molecules on the surface must attain certain energy. This energy is absorbed from the adjacent molecule’s heat energy. The excited surface molecules then evaporate as gas, leaving the surface cooler than the before because the previous temperature of the bulk has caused the evaporation. In short, the temperature of the bulk of liquid or the underneath surface is transferred to the surface of the liquid, which excites the surface molecules to cause evaporation. This causes the cooling of the remaining surfaces.
Additional information:
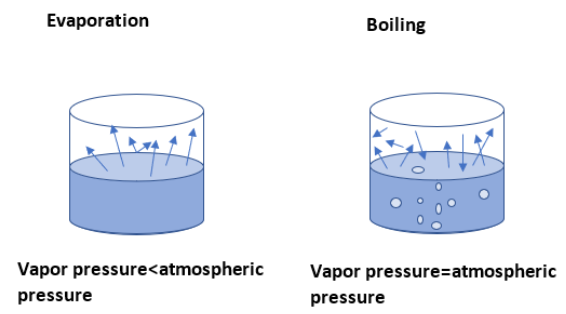
Boiling and evaporation are two different phenomena even though both involve the state change from liquid to gas. When the bulk of the liquid is heated by an external source with help of a burner, this vaporization that occurs is termed boiling. Evaporation is a self-occurring phenomenon and some factors like pressure, outside temperature, humidity etc. determines the rate of evaporation.
Note: Boiling does not cause any surface cooling, unlike Evaporation. During boiling, the vapour pressure overcomes the atmospheric pressure and bubbles will form. But, in the case of evaporation, bubbles won’t form since the atmospheric pressure is always greater than the vapour pressure. High pressure and higher room temperature can increase the evaporation rate. A humid climate slows down the rate of evaporation. Watering the rooftops and walls during summer is an application of the evaporation that is accompanied by cooling.
Complete step by step answer:
When atoms and molecules are in the liquid state, there is some interatomic attraction between the nearby molecules. To get transferred to the gaseous state without any extra heat supplied to the surface of the liquid, the liquid molecules on the surface must attain certain energy. This energy is absorbed from the adjacent molecule’s heat energy. The excited surface molecules then evaporate as gas, leaving the surface cooler than the before because the previous temperature of the bulk has caused the evaporation. In short, the temperature of the bulk of liquid or the underneath surface is transferred to the surface of the liquid, which excites the surface molecules to cause evaporation. This causes the cooling of the remaining surfaces.
Additional information:
Boiling and evaporation are two different phenomena even though both involve the state change from liquid to gas. When the bulk of the liquid is heated by an external source with help of a burner, this vaporization that occurs is termed boiling. Evaporation is a self-occurring phenomenon and some factors like pressure, outside temperature, humidity etc. determines the rate of evaporation.
Note: Boiling does not cause any surface cooling, unlike Evaporation. During boiling, the vapour pressure overcomes the atmospheric pressure and bubbles will form. But, in the case of evaporation, bubbles won’t form since the atmospheric pressure is always greater than the vapour pressure. High pressure and higher room temperature can increase the evaporation rate. A humid climate slows down the rate of evaporation. Watering the rooftops and walls during summer is an application of the evaporation that is accompanied by cooling.

Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




