
Fluorine exhibits only -1 oxidation state while iodine exhibits oxidation states of -1, +1, +3, +5 and +7. This is due to:
(A) fluorine being a gas.
(B) available d-orbitals in iodine.
(C) non-availability of d-orbitals in iodine.
(D) none of the above.
Answer
566.4k+ views
Hint: Fluorine and Iodine belongs to Group 17. They are commonly called as Halogens. Halogens are highly reactive nonmetals and also known as p-block elements.
Complete answer:
Oxidation state means degree of oxidation for an atom in a chemical compound. Oxidation state represented by an integer, which can be positive, negative or zero.
Fluorine and iodine are group 17 members.
There electronic configuration is
F = Atomic number = 9 = $1{s^2}2{s^2}2{p^5} = [He]2{s^2}2{p^5}$
I = Atomic number = 53 = $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^6}$
$ = [Kr]4{d^{10}}5{d^2}5{p^6}$
Ground state Electronic configuration of fluorine is
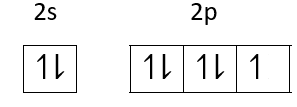
Fluorine is the most electronegative atom so it accepts electrons very easily and shows only -1 oxidation state.
Ground state electronic configuration of iodine is shown in the diagram below.
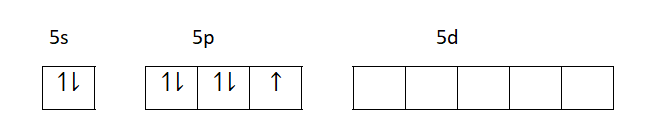
First excited state
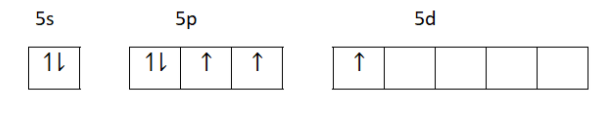
Second excited state

Third excited state
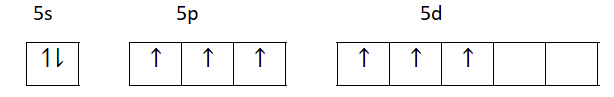
Therefore, iodine shows +1, +3, +5, +7 oxidation state apart from oxidation state -1 because of the presence of empty vacant d- orbitals which are not present in fluorine. So Fluorine does not show any higher oxidation state.
Note: Positive oxidation state is possible by excitation of outer s and p-orbitals into d-orbitals so that 3, 5 or 7 unpaired electrons are easily available for bonding. Elements which do not have vacant d orbitals can’t show higher oxidation state.
Complete answer:
Oxidation state means degree of oxidation for an atom in a chemical compound. Oxidation state represented by an integer, which can be positive, negative or zero.
Fluorine and iodine are group 17 members.
There electronic configuration is
F = Atomic number = 9 = $1{s^2}2{s^2}2{p^5} = [He]2{s^2}2{p^5}$
I = Atomic number = 53 = $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^6}$
$ = [Kr]4{d^{10}}5{d^2}5{p^6}$
Ground state Electronic configuration of fluorine is
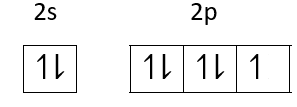
Fluorine is the most electronegative atom so it accepts electrons very easily and shows only -1 oxidation state.
Ground state electronic configuration of iodine is shown in the diagram below.

First excited state

Second excited state

Third excited state

Therefore, iodine shows +1, +3, +5, +7 oxidation state apart from oxidation state -1 because of the presence of empty vacant d- orbitals which are not present in fluorine. So Fluorine does not show any higher oxidation state.
Note: Positive oxidation state is possible by excitation of outer s and p-orbitals into d-orbitals so that 3, 5 or 7 unpaired electrons are easily available for bonding. Elements which do not have vacant d orbitals can’t show higher oxidation state.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




