
For a molecule with two like chiral carbon atoms, the number of optically active form is:
A. 4
B. 3
C. 1
D. 2
Answer
582.9k+ views
Hint: Chiral carbon atoms are those carbon which are attached with four different atoms. In the question itself it is told that there are two like chiral carbon atoms means they are symmetric in nature with each other. To calculate the no of optically active forms for n no of chiral atoms is ${{2}^{n-1}}$.
Complete step by step answer:
From your chemistry lessons you have read about the chirality and optical activity.
Chirality is a property of an element which is shown by the arrangement of elements around the carbon atom. And its result from its structure. Chiral carbon atoms have four different atoms attached to it and all the chiral atoms are asymmetric in nature.
Chiral atoms do not have any plane of symmetry and have non-superimposable nature.
All chiral molecules with non-superimposable mirror images are Enantiomers.
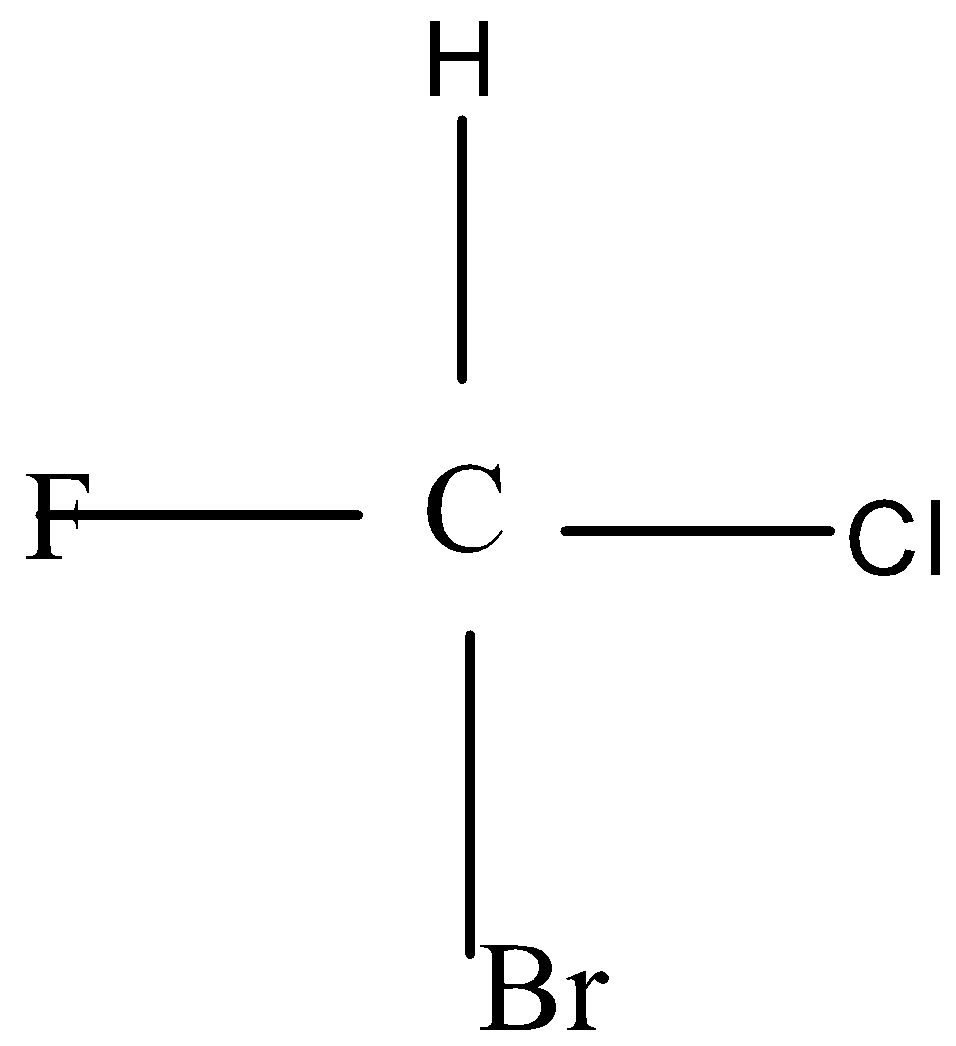
This figure is showing that carbon is attached with four different atoms.
Optical activity is a type of macroscopic property which is a collection of a molecule that arises in the way they interact with light. And compounds which are optically active always have chiral molecules.
Two like chiral chiral atoms means the two carbon that are attached on each side of a compound have the same four different groups attached with the carbons. These two like chiral carbon show symmetry with each other but the overall compound is asymmetric and optically active in nature because the orientation of carbon atoms on each side of the compound are different.
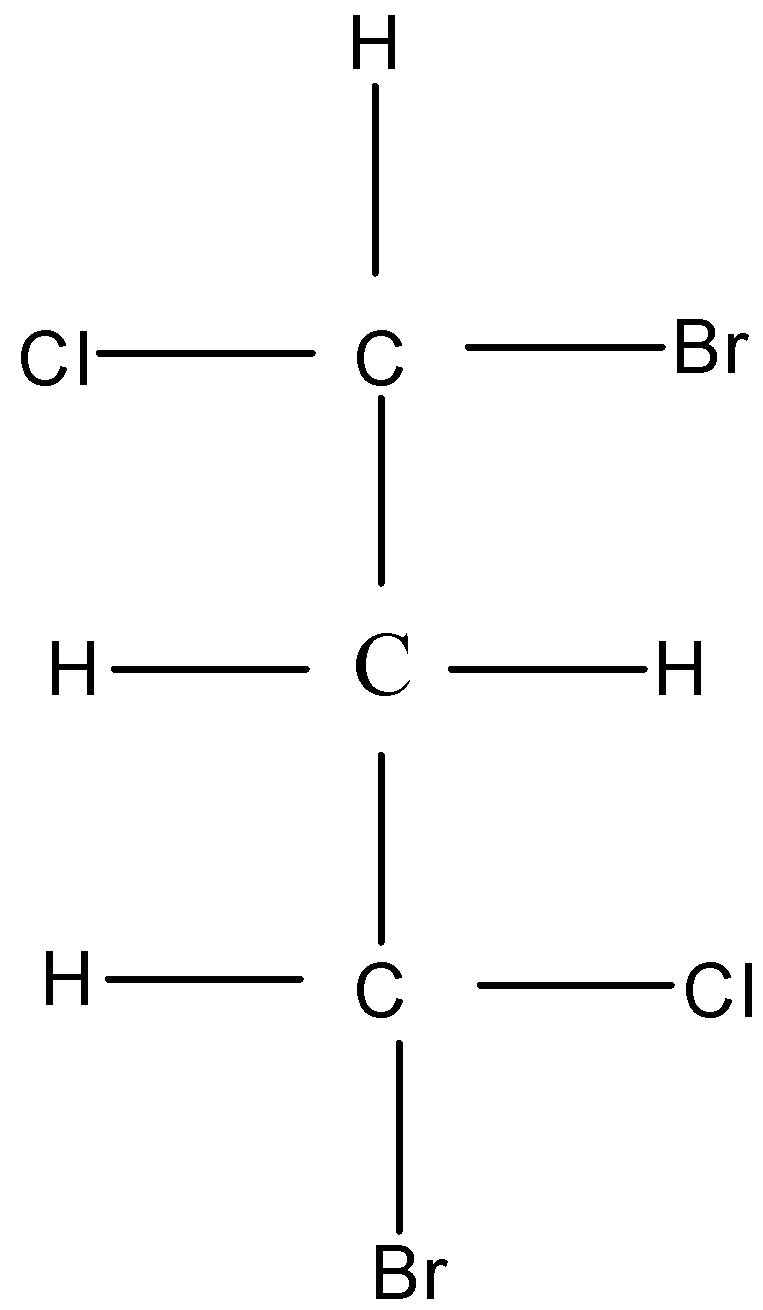
Like chiral carbon atoms are shown in this figure.
Now, the racemic mixture are those mixtures which have equal amounts of right and left handed enantiomers (chiral molecules). Racemic mixtures are also optically active.
To find the no. of optically active forms of molecule having n no of chiral carbon we use formula ${{2}^{n-1}}$,Where n is the no of chiral carbon in a compound.
Here 2 like chiral carbon atoms are given,
So, n = 2
Therefore, no of optically active form will be = ${{2}^{n-1}}={{2}^{2-1}}=2$
So, the correct answer is “Option D”.
Note: All chiral atoms are optically active and do not show symmetry and have non-superimposable mirror image. If we have to find the no of configurational isomers with n no. of chiral atoms then we will use the formula of 2n. Enantiomers have chiral centers.
Complete step by step answer:
From your chemistry lessons you have read about the chirality and optical activity.
Chirality is a property of an element which is shown by the arrangement of elements around the carbon atom. And its result from its structure. Chiral carbon atoms have four different atoms attached to it and all the chiral atoms are asymmetric in nature.
Chiral atoms do not have any plane of symmetry and have non-superimposable nature.
All chiral molecules with non-superimposable mirror images are Enantiomers.

This figure is showing that carbon is attached with four different atoms.
Optical activity is a type of macroscopic property which is a collection of a molecule that arises in the way they interact with light. And compounds which are optically active always have chiral molecules.
Two like chiral chiral atoms means the two carbon that are attached on each side of a compound have the same four different groups attached with the carbons. These two like chiral carbon show symmetry with each other but the overall compound is asymmetric and optically active in nature because the orientation of carbon atoms on each side of the compound are different.

Like chiral carbon atoms are shown in this figure.
Now, the racemic mixture are those mixtures which have equal amounts of right and left handed enantiomers (chiral molecules). Racemic mixtures are also optically active.
To find the no. of optically active forms of molecule having n no of chiral carbon we use formula ${{2}^{n-1}}$,Where n is the no of chiral carbon in a compound.
Here 2 like chiral carbon atoms are given,
So, n = 2
Therefore, no of optically active form will be = ${{2}^{n-1}}={{2}^{2-1}}=2$
So, the correct answer is “Option D”.
Note: All chiral atoms are optically active and do not show symmetry and have non-superimposable mirror image. If we have to find the no of configurational isomers with n no. of chiral atoms then we will use the formula of 2n. Enantiomers have chiral centers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




