
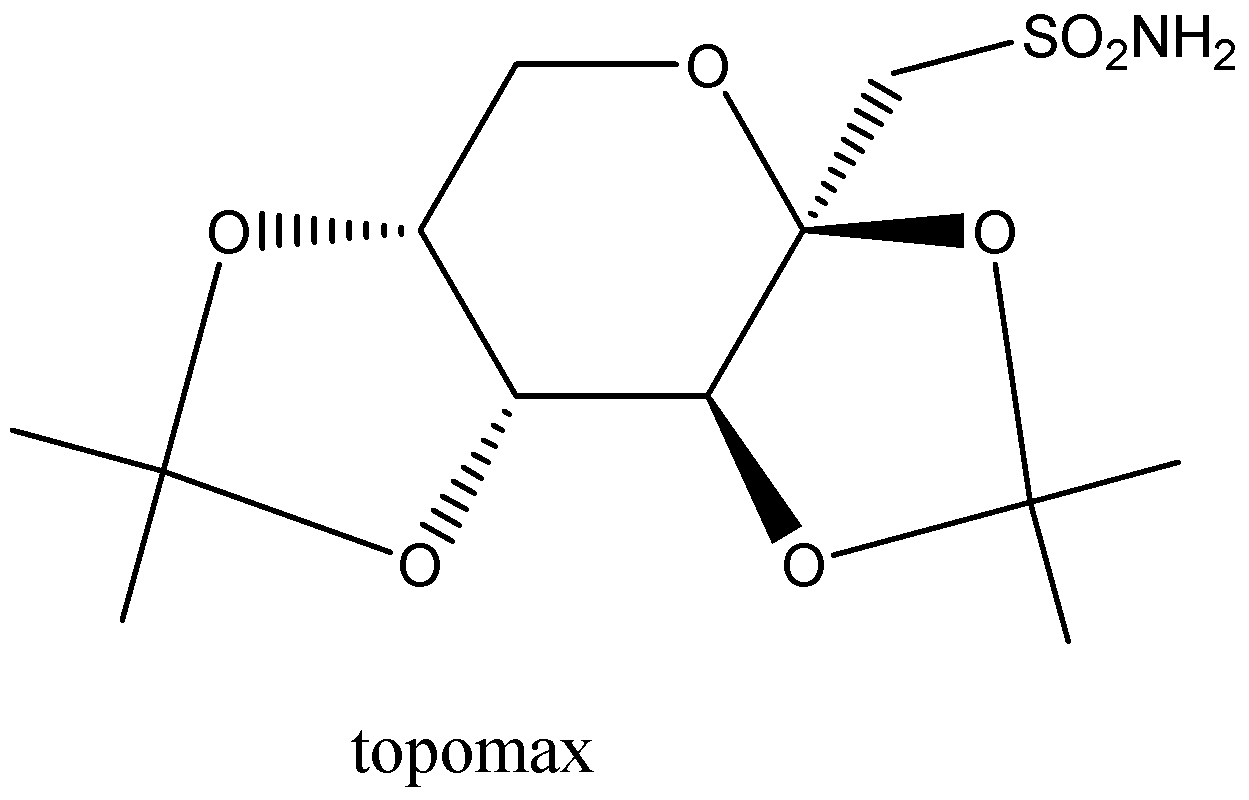
For the given pharmaceutical compounds, identify all stereogenic (i.e., asymmetric carbon atoms) and label the configuration of each as being either \[\left( R \right)\] or\[\left( S \right)\].

Answer
592.5k+ views
Hint: Firstly, let’s see that in the case of a stereogenic center we need to focus on the valencies that are subjected to the carbon atom in particular with respect to different groups. After that our aim is to keep a check on the priority orders of the atoms from the center and then compare it to the center. At last, we need to consider the configuration.
Complete step by step answer:
We can also call a stereogenic center as a stereocenter which is a point in a molecule and not in an atom. These molecules are attached with different types of substituents that are responsible for interchanging any two substituents from where you can easily reach to the stereoisomer.
Now, on the given Topamax compound we will see that there are four valencies that belong to the carbon atom and each of the carbon valencies is related to a different group. Then, let’s keep in mind that whenever there are different groups of valencies then we need to arrange them properly in a clockwise direction.
After that, we will arrange them in the increasing order of their priority in the clockwise direction with respect to the center, then it will show us R-configuration. And all the groups that are arranged in an anti-clockwise direction will have S-configuration.
Here, we have easily pointed out that there are two each R and S-configurations on both sides.

Note:
We must know the difference between the DL and RS configuration. Therefore, D and L describe the direction in which polarized light is rotated by the molecule. But R and S give you specific information about the position of groups on a chiral center.
Complete step by step answer:
We can also call a stereogenic center as a stereocenter which is a point in a molecule and not in an atom. These molecules are attached with different types of substituents that are responsible for interchanging any two substituents from where you can easily reach to the stereoisomer.
Now, on the given Topamax compound we will see that there are four valencies that belong to the carbon atom and each of the carbon valencies is related to a different group. Then, let’s keep in mind that whenever there are different groups of valencies then we need to arrange them properly in a clockwise direction.
After that, we will arrange them in the increasing order of their priority in the clockwise direction with respect to the center, then it will show us R-configuration. And all the groups that are arranged in an anti-clockwise direction will have S-configuration.
Here, we have easily pointed out that there are two each R and S-configurations on both sides.

Note:
We must know the difference between the DL and RS configuration. Therefore, D and L describe the direction in which polarized light is rotated by the molecule. But R and S give you specific information about the position of groups on a chiral center.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




