
For the solution of the gases w, x, y, and z in the water at $\text{ 298 K }$ , Henry’s law constant $\text{ }\left( {{\text{k}}_{\text{H}}} \right)\text{ }$is $\text{ 0}\text{.5 }$ , $\text{ 2}\text{.0 }$ , $\text{ 35 }$ and $\text{ 40 }$ $\text{ kbar }$ , respectively. The correct plot for the given data is :
(A)
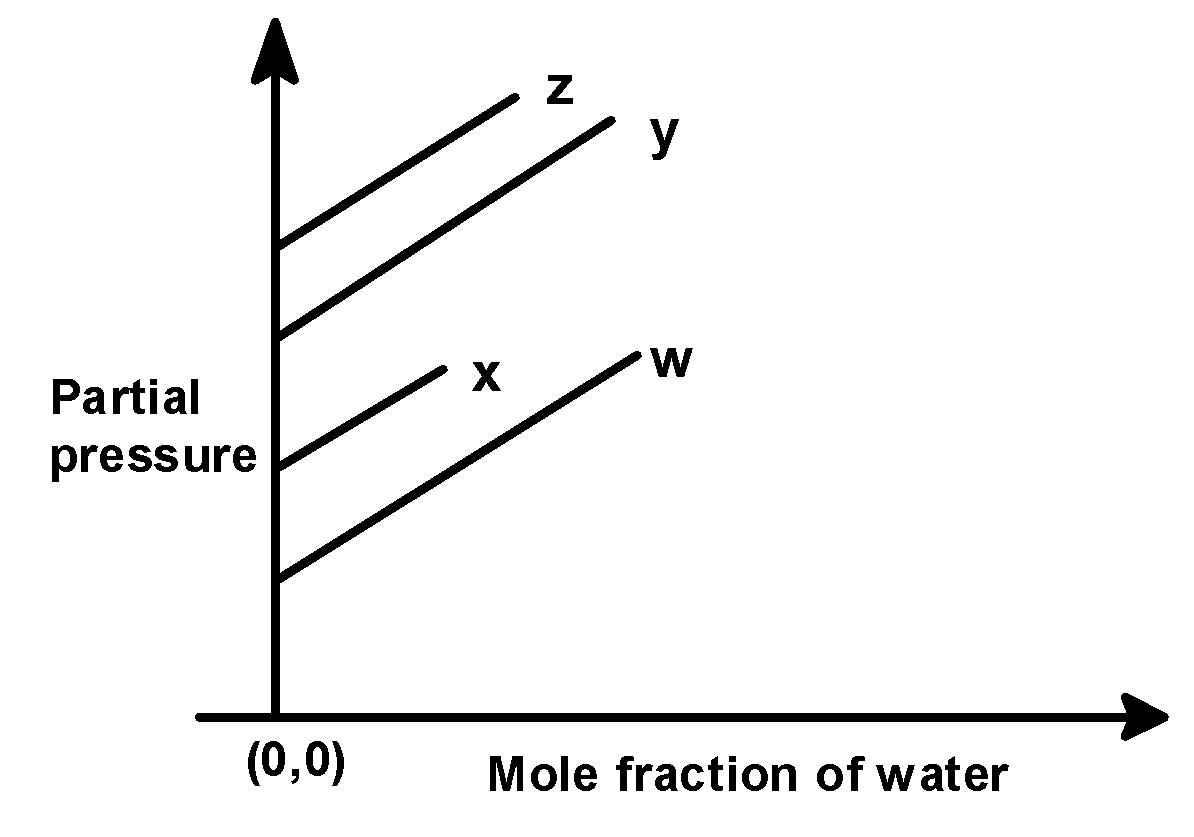
(B)
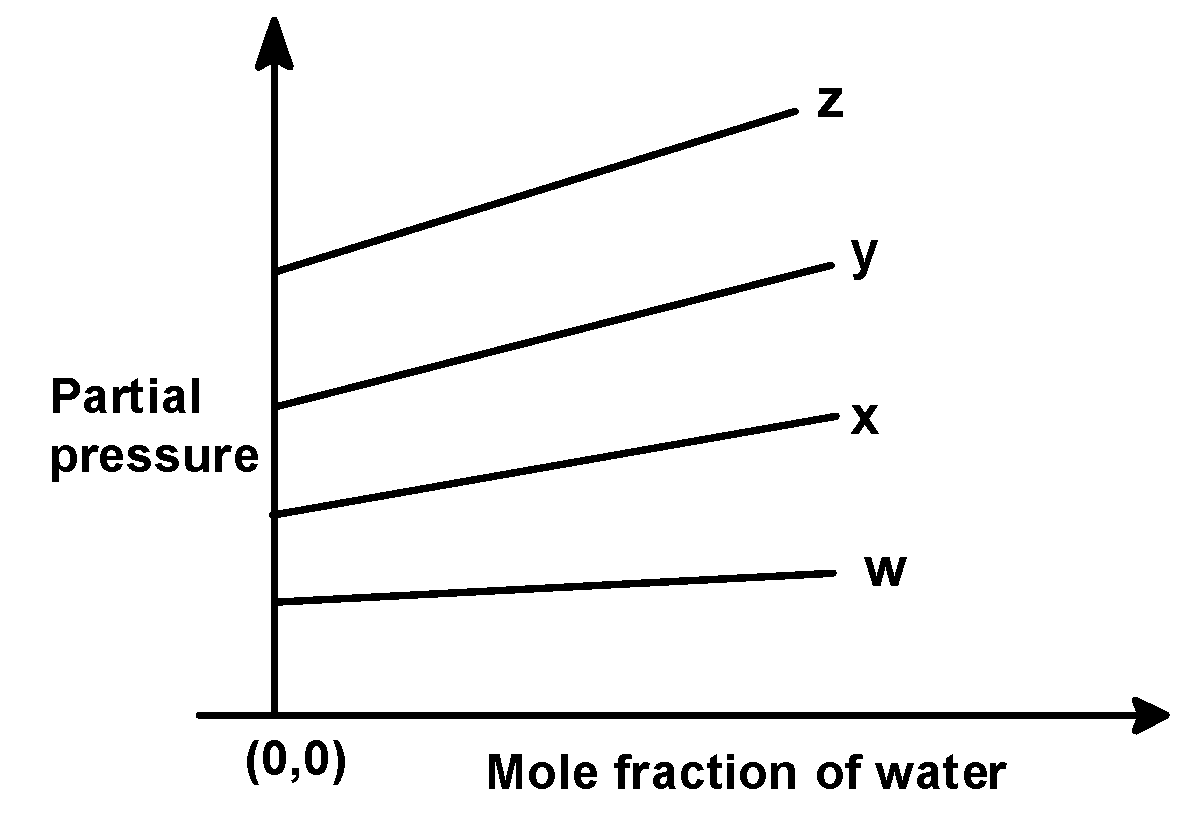
(C)
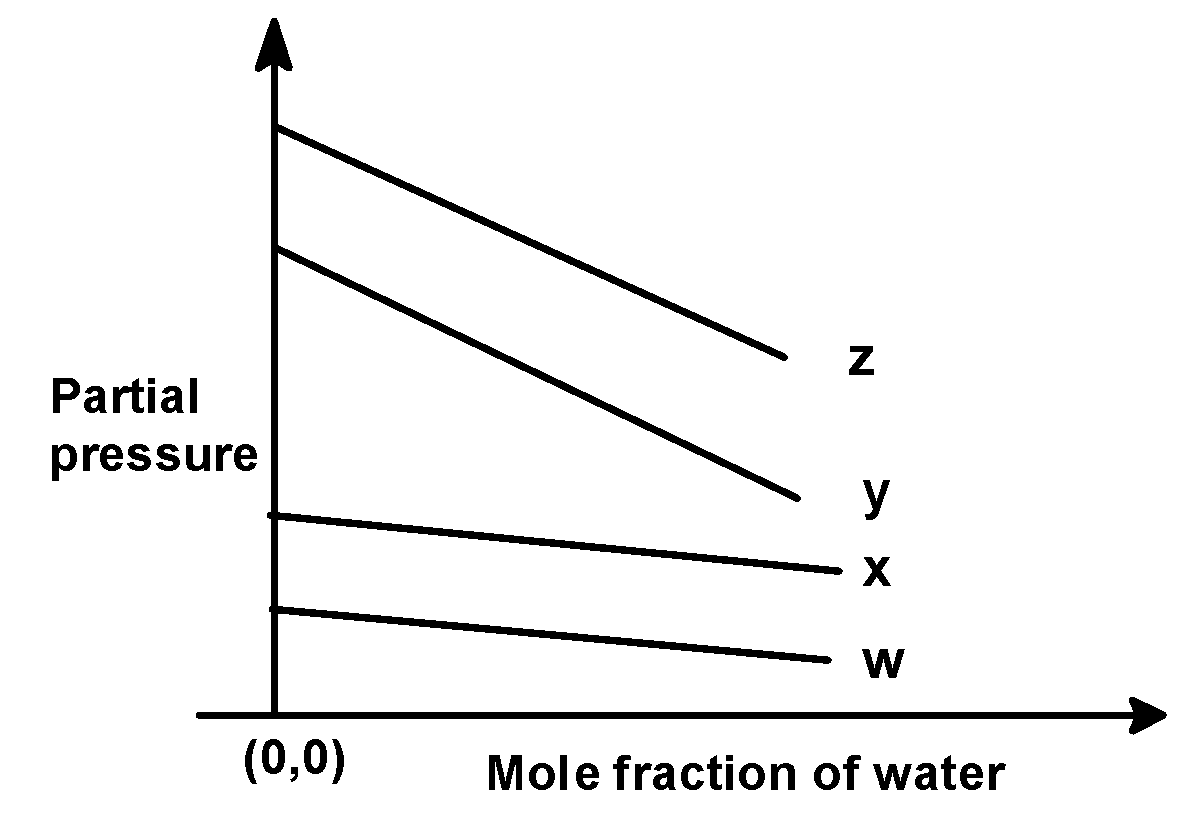
(D)
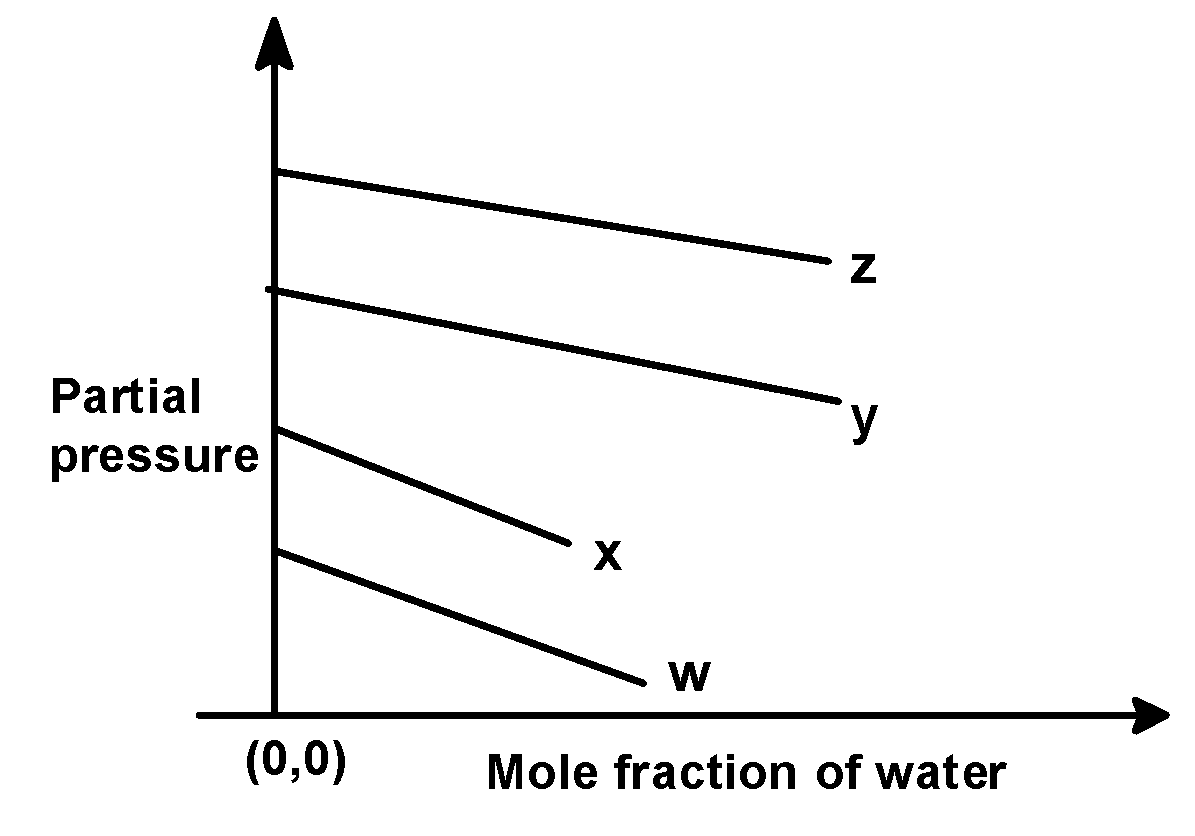




Answer
579k+ views
Hint: The pressure has a remarkable effect on the solubility of the gas. The relation between the partial pressure and mole fraction of a gas solute is given as,
$\text{ }{{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}\text{ }\times \text{ }{{\chi }_{\text{gas}}}\text{ }$ (1)
Where $\text{ }{{\chi }_{\text{gas}}}\text{ }$ the mole fraction of solute and$\text{ }{{\text{k}}_{\text{H}}}\text{ }$ is henry’s constant. The plot of the mole fraction of gas against the partial pressure is a straight line and follows the straight-line equation$\text{ y = mx + C }$.
Complete step by step answer:
-The solubility of gases at a given temperature increases directly as the pressure. This conclusion forms a basis of what is known as Henry’s law, which may be stated as below.
-The mass of a gas dissolved per unit volume of a solvent is proportional to the pressure of the gas in equilibrium with the solution at a constant temperature.
-The henry law can be alternatively stated as the pressure of the gas over the solution in which the gas is dissolved is proportional to the mole fraction of the gas dissolved in solution. That is,
$\text{ }{{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}\text{ }\times \text{ }{{\chi }_{\text{gas}}}\text{ }$ (1)
Where $\text{ }{{\chi }_{\text{gas}}}\text{ }$ the mole fraction of solute and$\text{ }{{\text{k}}_{\text{H}}}\text{ }$ is henry’s constant.
-If gas is dissolved in the water then the sum of the mole fraction of the water as gas would be equal to 1. That is,
$\begin{align}
& \text{ }{{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ + }{{\chi }_{\text{gas}}}\text{ = 1} \\
& \text{or }{{\chi }_{\text{gas}}}\text{ }=\text{ 1}-{{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ } \\
\end{align}$
Thus, equation (1) would become,
$\begin{align}
& \text{ }{{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}\text{ }\times \text{ }\left( \text{1}-{{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}} \right) \\
& \Rightarrow {{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}-{{\text{k}}_{\text{H}}}\times {{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}} \\
& \Rightarrow {{\text{p}}_{\text{gas}}}\text{ = }\left( -{{\text{k}}_{\text{H}}} \right)\times {{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ + }{{\text{k}}_{\text{H}}}\text{ } \\
\end{align}$
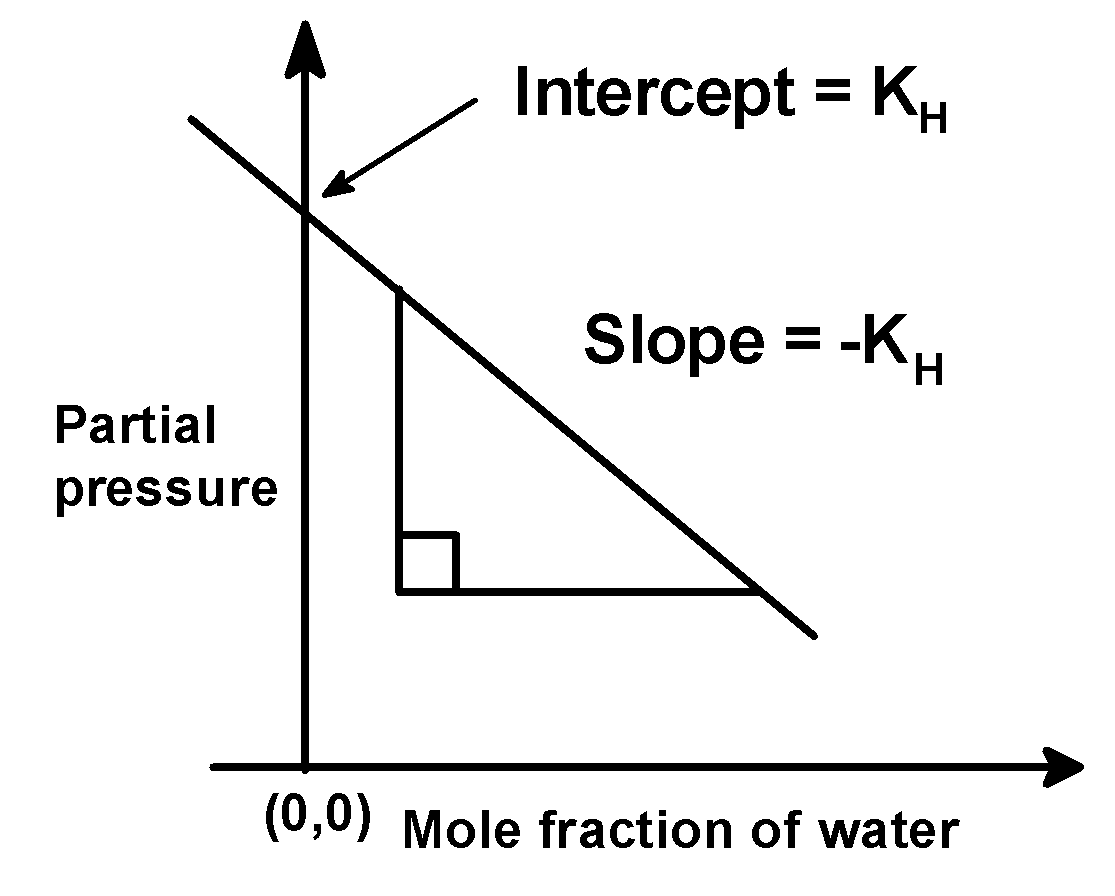
The equation $\text{ }{{\text{p}}_{\text{gas}}}\text{ = }\left( -{{\text{k}}_{\text{H}}} \right)\times {{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ + }{{\text{k}}_{\text{H}}}\text{ }$ (2)
Seems similar to the equation of the straight line which is,
$\text{ y = mx + C }$ (3)
Where m is the slope and C is the intercept.
On comparing (2) and (3) we get that,
The slope is $\text{ }\left( -{{\text{k}}_{\text{H}}} \right)\text{ }$ an intercept is$\text{ }\left( {{\text{k}}_{\text{H}}} \right)\text{ }$.
In the problem, we have given the following intercepts,
$\text{ w = 0}\text{.5 }$, $\text{ x = 2}\text{.0 }$, $\text{ y = 35 }$and $\text{ z = 40 }$kilo bar. Thus the increasing order of intercepts for the given plot would be written as,
$\text{ w }<\text{ x }<\text{ y }<\text{ z }$
This order is seen in plot C).
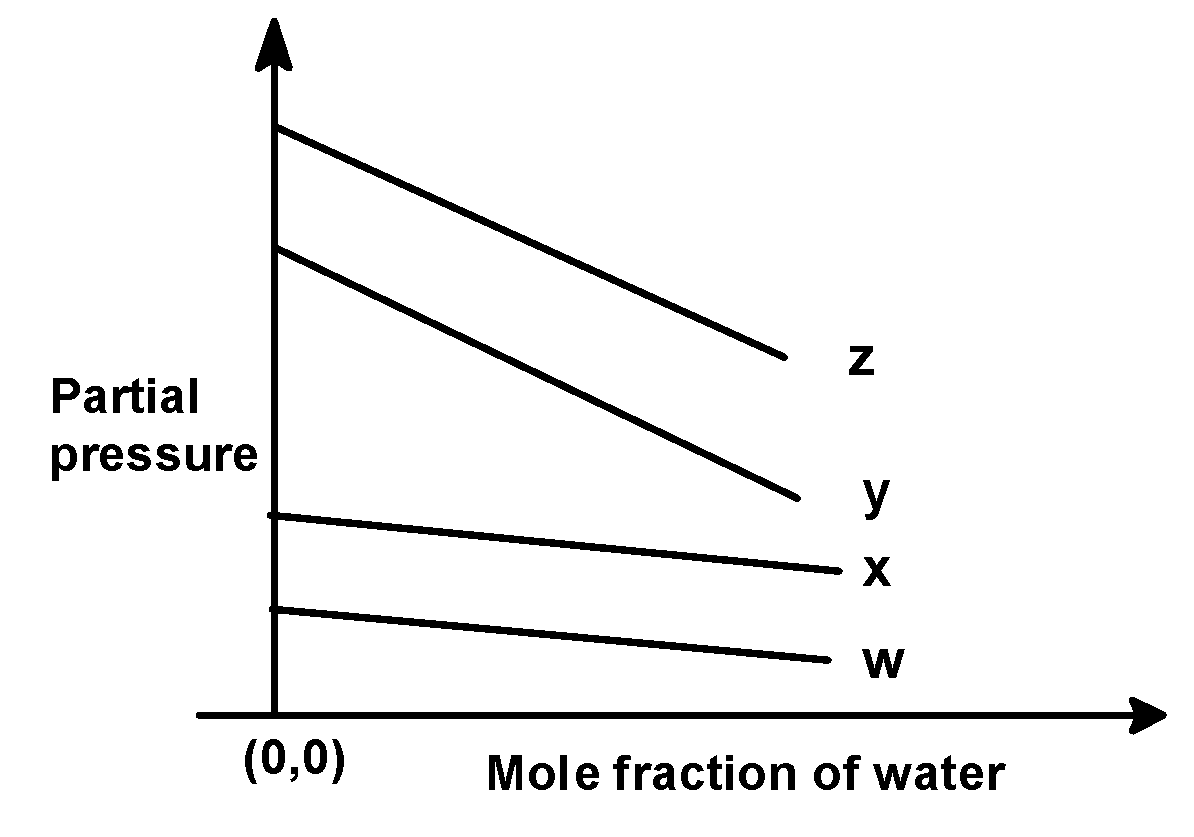
Hence, (C) is the correct option.
Note: Note that, if a gas obeys a henry's law then the graph obtained by plotting solubility of gas (i.e. Mole fraction of solute) against the pressure at the constant pressure would be a straight line.
The gas obeys the henry's law provided,
1. Pressure is not too high
2. Temperature is not too low
3. Gas is not highly soluble and does not dissociate or associate in the solution.
$\text{ }{{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}\text{ }\times \text{ }{{\chi }_{\text{gas}}}\text{ }$ (1)
Where $\text{ }{{\chi }_{\text{gas}}}\text{ }$ the mole fraction of solute and$\text{ }{{\text{k}}_{\text{H}}}\text{ }$ is henry’s constant. The plot of the mole fraction of gas against the partial pressure is a straight line and follows the straight-line equation$\text{ y = mx + C }$.
Complete step by step answer:
-The solubility of gases at a given temperature increases directly as the pressure. This conclusion forms a basis of what is known as Henry’s law, which may be stated as below.
-The mass of a gas dissolved per unit volume of a solvent is proportional to the pressure of the gas in equilibrium with the solution at a constant temperature.
-The henry law can be alternatively stated as the pressure of the gas over the solution in which the gas is dissolved is proportional to the mole fraction of the gas dissolved in solution. That is,
$\text{ }{{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}\text{ }\times \text{ }{{\chi }_{\text{gas}}}\text{ }$ (1)
Where $\text{ }{{\chi }_{\text{gas}}}\text{ }$ the mole fraction of solute and$\text{ }{{\text{k}}_{\text{H}}}\text{ }$ is henry’s constant.
-If gas is dissolved in the water then the sum of the mole fraction of the water as gas would be equal to 1. That is,
$\begin{align}
& \text{ }{{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ + }{{\chi }_{\text{gas}}}\text{ = 1} \\
& \text{or }{{\chi }_{\text{gas}}}\text{ }=\text{ 1}-{{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ } \\
\end{align}$
Thus, equation (1) would become,
$\begin{align}
& \text{ }{{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}\text{ }\times \text{ }\left( \text{1}-{{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}} \right) \\
& \Rightarrow {{\text{p}}_{\text{gas}}}\text{ = }{{\text{k}}_{\text{H}}}-{{\text{k}}_{\text{H}}}\times {{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}} \\
& \Rightarrow {{\text{p}}_{\text{gas}}}\text{ = }\left( -{{\text{k}}_{\text{H}}} \right)\times {{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ + }{{\text{k}}_{\text{H}}}\text{ } \\
\end{align}$
The equation $\text{ }{{\text{p}}_{\text{gas}}}\text{ = }\left( -{{\text{k}}_{\text{H}}} \right)\times {{\chi }_{{{\text{H}}_{\text{2}}}\text{O}}}\text{ + }{{\text{k}}_{\text{H}}}\text{ }$ (2)
Seems similar to the equation of the straight line which is,
$\text{ y = mx + C }$ (3)
Where m is the slope and C is the intercept.
On comparing (2) and (3) we get that,
The slope is $\text{ }\left( -{{\text{k}}_{\text{H}}} \right)\text{ }$ an intercept is$\text{ }\left( {{\text{k}}_{\text{H}}} \right)\text{ }$.
In the problem, we have given the following intercepts,
$\text{ w = 0}\text{.5 }$, $\text{ x = 2}\text{.0 }$, $\text{ y = 35 }$and $\text{ z = 40 }$kilo bar. Thus the increasing order of intercepts for the given plot would be written as,
$\text{ w }<\text{ x }<\text{ y }<\text{ z }$
This order is seen in plot C).

Hence, (C) is the correct option.
Note: Note that, if a gas obeys a henry's law then the graph obtained by plotting solubility of gas (i.e. Mole fraction of solute) against the pressure at the constant pressure would be a straight line.

The gas obeys the henry's law provided,
1. Pressure is not too high
2. Temperature is not too low
3. Gas is not highly soluble and does not dissociate or associate in the solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




