
What is the formula of hydrochloric acid?
A. $HCl$
B. $HC{{l}_{2}}$
C. ${{H}_{2}}C{{l}_{2}}$
D. $HC{{l}_{3}}$
Answer
595.8k+ views
Hint: As the name suggests the acid consists of hydrogen and chlorine atoms. Only the number of atoms used to form hydrochloric acid has to be figured out. A compound in form when there is a bond between them, it can be ionic or covalent bond.
Complete step by step answer:
The connections between atoms in a molecule which binds them together are called Chemical bonds. For the molecule mentioned in the question the chemical bond is covalent bond. A covalent bond is formed due to interaction between two atoms, which involves the sharing of one or more electrons to help each other satisfy the octet rule. This interaction generally exists between two non-metals.
As we know, the atomic number of hydrogen is 1 and in the outermost shell it contains 1 electron. Where chlorine has its atomic number 17 and in the outermost shell have 1 electron (the outermost shell of an atom can accommodate a maximum of 8 electrons).
So, by sharing their electrons they make themselves balanced and make their configuration of 8 complete.
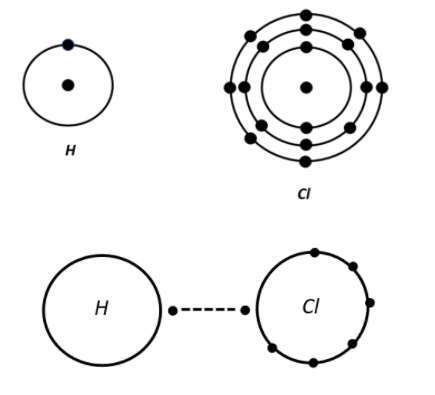
Hydrochloric acid (HCl), is also known as muriatic acid. It is a colourless corrosive, strong mineral acid. It is a colourless and pungent smelling inorganic compound. Hydrochloric acid is a simple diatomic molecule and is found in both aqueous and gaseous forms. A polarized covalent bond binds the electronegative chlorine atom and the hydrogen atom.
So, the correct option is (A).
Note: A compound can only form when there is an atom to balance their configuration with their valency. Chemical bonds of different types try not to get confused between them.
Complete step by step answer:
The connections between atoms in a molecule which binds them together are called Chemical bonds. For the molecule mentioned in the question the chemical bond is covalent bond. A covalent bond is formed due to interaction between two atoms, which involves the sharing of one or more electrons to help each other satisfy the octet rule. This interaction generally exists between two non-metals.
As we know, the atomic number of hydrogen is 1 and in the outermost shell it contains 1 electron. Where chlorine has its atomic number 17 and in the outermost shell have 1 electron (the outermost shell of an atom can accommodate a maximum of 8 electrons).
So, by sharing their electrons they make themselves balanced and make their configuration of 8 complete.

Hydrochloric acid (HCl), is also known as muriatic acid. It is a colourless corrosive, strong mineral acid. It is a colourless and pungent smelling inorganic compound. Hydrochloric acid is a simple diatomic molecule and is found in both aqueous and gaseous forms. A polarized covalent bond binds the electronegative chlorine atom and the hydrogen atom.
So, the correct option is (A).
Note: A compound can only form when there is an atom to balance their configuration with their valency. Chemical bonds of different types try not to get confused between them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




