
Frenkel defect is caused due to:
A) An ion missing from the normal lattice site creating a vacancy.
B) An extra positive ion occupying an interstitial position in the lattice.
C) An extra negative ion occupying an interstitial position in the lattice.
D) The shift of a position ion from its normal lattice to an interstitial site.
Answer
564.9k+ views
Hint:Solid crystals show some kind of defects. Some units of the crystals may have one or more atoms in their crystal, this gives rise to imperfections in the crystal also known as defects of crystals. There are many types of defects such as line defect, point defect, planar defect etc. here we will talk about Frenkel defect which is a point defect.
Complete solution:
We know that the positive ions (cations) are smaller than the negative ions (anions)
So the Frenkel defect is a defect in which a positive ion(cation) leaves its place in the lattice thereby occupying a nearby interstitial position. This will create a vacancy in the lattice.
Frenkel defect is caused when the size of the anion is very large as compared to the size of the cation. Because of this size difference between the ions, the ions prefer to occupy interstitial positions in the lattice. Thus, Frenkel defects are shown by those ionic solids which have large size differences between cation and anion.
Example: $NaCl,ZnS,Agl$ etc.
Here is a depiction of a solid crystal showing Frenkel defect:
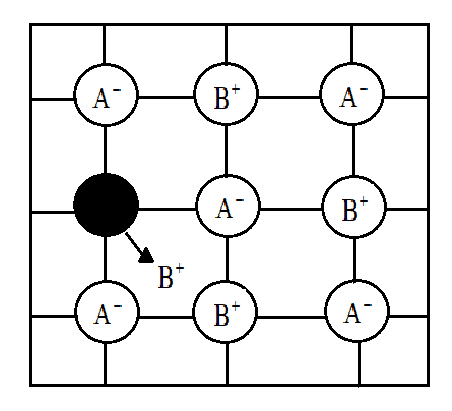
Therefore, from the above explanation we can say that the correct option is (D).
Note: As we see here that the number of atoms before and after the defect remains the same hence the density also remains the same. So, the density, mass and volume of the ionic crystal remain the same even after the Frenkel defect. Also, as the number of cations and anions do not change so the ionic compound remains neutral in nature.
Complete solution:
We know that the positive ions (cations) are smaller than the negative ions (anions)
So the Frenkel defect is a defect in which a positive ion(cation) leaves its place in the lattice thereby occupying a nearby interstitial position. This will create a vacancy in the lattice.
Frenkel defect is caused when the size of the anion is very large as compared to the size of the cation. Because of this size difference between the ions, the ions prefer to occupy interstitial positions in the lattice. Thus, Frenkel defects are shown by those ionic solids which have large size differences between cation and anion.
Example: $NaCl,ZnS,Agl$ etc.
Here is a depiction of a solid crystal showing Frenkel defect:

Therefore, from the above explanation we can say that the correct option is (D).
Note: As we see here that the number of atoms before and after the defect remains the same hence the density also remains the same. So, the density, mass and volume of the ionic crystal remain the same even after the Frenkel defect. Also, as the number of cations and anions do not change so the ionic compound remains neutral in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




