
Fructose gives positive Fehling’s solution test.
A.True
B.False
Answer
573.6k+ views
Hint: Aldehydes which are present in the sugar gives positive Fehling’s test. Fehling’s test is a chemical test which is generally used to distinguish between aldehydes and ketone functional groups.
Complete answer:
The sugars which acts as a reducing agent in the alkaline solution is known as reducing sugars. Fehling’s solution is a mixture of copper sulphate, potassium sodium tartrate and sodium hydroxide. It is used to differentiate between the water soluble carbohydrates that are aldehydes and the ketonic functional group and acts as a test for the reducing sugars.
In the basic medium, fructose shows keto enol tautomerism and thus isomerizes to glucose due to which fructose gives a positive Fehling’s test. This is shown below:
\[D-fructoseenol\text{ intermediate}D-glu\cos e\]
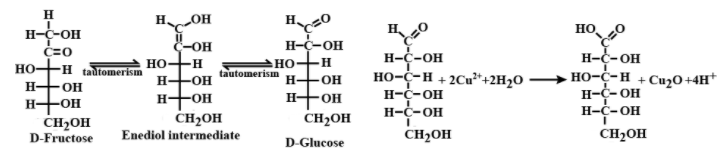
Hence the correct answer is (A) i.e. Fructose given positive Fehling’s solution test is a true statement.
Additional information:
Fehling’s solution is prepared by mixing Fehling’s A and Fehling’s B. this solution acts as an oxidizing agent. Due to the presence of copper the solution gives blue color which on reaction with the aldehyde group turns the copper ions to copper oxide which appears as red precipitate.
The sugar having free aldehyde groups gets reduced but the one without the aldehyde group gives a negative test. The reaction involved is mentioned below:
$RCHO+2C{{u}^{2+}}+5O{{H}^{-}}\to RCO{{O}^{-}}+C{{u}_{2}}O+3{{H}_{2}}O$
Note:
The Fehling’s test is sensitive enough because 1 mg of glucose will be sufficient to produce the characteristic red color of the compound. This test is used to differentiate between the reducing sugars and non reducing sugars. And one more important point is that this test will turn out positive only when a free aldehyde group is present.
Complete answer:
The sugars which acts as a reducing agent in the alkaline solution is known as reducing sugars. Fehling’s solution is a mixture of copper sulphate, potassium sodium tartrate and sodium hydroxide. It is used to differentiate between the water soluble carbohydrates that are aldehydes and the ketonic functional group and acts as a test for the reducing sugars.
In the basic medium, fructose shows keto enol tautomerism and thus isomerizes to glucose due to which fructose gives a positive Fehling’s test. This is shown below:
\[D-fructoseenol\text{ intermediate}D-glu\cos e\]

Hence the correct answer is (A) i.e. Fructose given positive Fehling’s solution test is a true statement.
Additional information:
Fehling’s solution is prepared by mixing Fehling’s A and Fehling’s B. this solution acts as an oxidizing agent. Due to the presence of copper the solution gives blue color which on reaction with the aldehyde group turns the copper ions to copper oxide which appears as red precipitate.
The sugar having free aldehyde groups gets reduced but the one without the aldehyde group gives a negative test. The reaction involved is mentioned below:
$RCHO+2C{{u}^{2+}}+5O{{H}^{-}}\to RCO{{O}^{-}}+C{{u}_{2}}O+3{{H}_{2}}O$
Note:
The Fehling’s test is sensitive enough because 1 mg of glucose will be sufficient to produce the characteristic red color of the compound. This test is used to differentiate between the reducing sugars and non reducing sugars. And one more important point is that this test will turn out positive only when a free aldehyde group is present.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




