
Geometry of ammonia molecule and the hybridisation involved in it are:
A. $s{p^3} - $hybridisation and tetrahedral geometry
B. $s{p^3} - $hybridisation and distorted tetrahedral geometry
C. $s{p^3} - $hybridisation and triangular geometry
D. none of above
Answer
598.8k+ views
Hint: Ammonia is also called nitrogen trihydride. Its chemical formula is $N{H_3}$ and molar mass $17.031$ gram per mole. It is the simplest inorganic base. It is found in rainwater, volcanic areas and in the atmosphere. It occurs naturally in the body and secreted by kidneys so that access of acid in the kidney can be neutralized. It is a colourless gas with sharp odour. It combines with various acids to form ammonium salts.
Complete answer:
The ammonia molecules have a distorted tetrahedral shape where nitrogen connects with three hydrogen atoms. Here nitrogen also has a lone pair of electrons which makes it a base . We know that geometry of molecules depends on the number of bond pairs and lone pairs of electrons.. The structure of ammonia is given below:
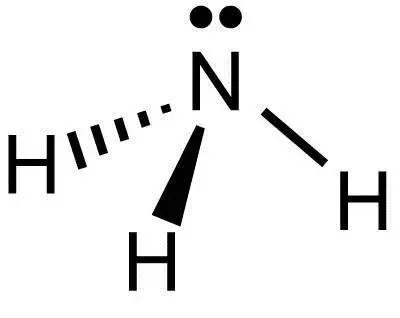
Ammonia molecule has three bond pairs and one lone pair so it has $s{p^3}$ hybridisation. The distorted tetrahedral shape of ammonia is because of overlap of three hydrogen atoms and three $s{p^3}$ hybrid orbitals of nitrogen whereas the fourth $s{p^3}$ hybrid orbital is involved by lone pairs. Hence here option B is correct. That is Geometry of the ammonia molecule and the hybridisation involved in it are $s{p^3} - $ hybridisation and distorted tetrahedral.
Note: Ammonia can form hydrogen bonds as it is a polar molecule. Liquid ammonia is an important non-aqueous solvent. It is the precursor to important compounds such as amino acids, phenol, urea, hydrogen cyanide, soda ash, acrylonitrile and many others. It is able to absorb a substantial amount of heat from the surroundings. It is useful in the manufacture of explosives ,dyes ,fabrics, pesticides and plastics.
Complete answer:
The ammonia molecules have a distorted tetrahedral shape where nitrogen connects with three hydrogen atoms. Here nitrogen also has a lone pair of electrons which makes it a base . We know that geometry of molecules depends on the number of bond pairs and lone pairs of electrons.. The structure of ammonia is given below:

Ammonia molecule has three bond pairs and one lone pair so it has $s{p^3}$ hybridisation. The distorted tetrahedral shape of ammonia is because of overlap of three hydrogen atoms and three $s{p^3}$ hybrid orbitals of nitrogen whereas the fourth $s{p^3}$ hybrid orbital is involved by lone pairs. Hence here option B is correct. That is Geometry of the ammonia molecule and the hybridisation involved in it are $s{p^3} - $ hybridisation and distorted tetrahedral.
Note: Ammonia can form hydrogen bonds as it is a polar molecule. Liquid ammonia is an important non-aqueous solvent. It is the precursor to important compounds such as amino acids, phenol, urea, hydrogen cyanide, soda ash, acrylonitrile and many others. It is able to absorb a substantial amount of heat from the surroundings. It is useful in the manufacture of explosives ,dyes ,fabrics, pesticides and plastics.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




