
Give an example of each homopolymer and copolymer.
Answer
587.7k+ views
Hint: Polymer obtained from only one type of monomer unit is called a homopolymer. The polymer obtained by the simple addition of more than one monomer unit or condensation(release of material during the reaction) of more than one type of monomer unit having bi or more functional groups is called a copolymer.
Complete step by step solution:
A polymer is a substance or material consisting of very large molecules, or macromolecules, composed of many repeating subunits. Due to their different properties, both synthetic and natural polymers play essential and important roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to cell structure and function. Polymers, both natural and synthetic, are created via polymerisation of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties, including toughness, high elasticity and a tendency to form amorphous structures rather than crystals.
The polymer obtained from only one type of monomer unit is called a homopolymer. The polymer obtained by the simple addition of more than one monomer unit or condensation(release of material during the reaction) of more than one type of monomer unit having bi or more functional groups is called a copolymer.
On the basis of used monomer polymer can be divided into two categories:
a) Homopolymer b) Copolymer
PVC (polyvinyl chloride) is a homopolymer.
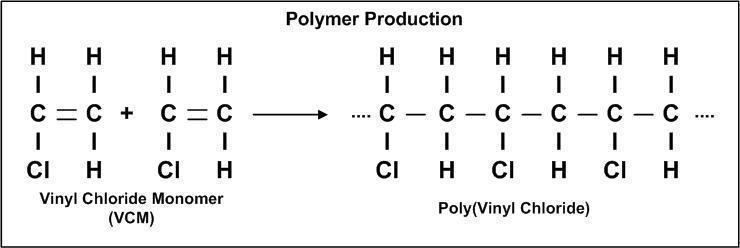
Polyvinyl chloride is produced in an additional polymerisation reaction using the chloroethene (vinyl chloride) monomer. This polymerisation reaction proceeds by a free-radical mechanism.
Nylon 6,6 is a copolymer.
Nylon -6,6 is synthesized by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/carboxylate mixture. The nylon salt goes into a reaction vessel where the polymerization process takes place either in batches or continuously.
Note: Homopolymer can be formed simply an additional reaction but copolymer not.
Complete step by step solution:
A polymer is a substance or material consisting of very large molecules, or macromolecules, composed of many repeating subunits. Due to their different properties, both synthetic and natural polymers play essential and important roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to cell structure and function. Polymers, both natural and synthetic, are created via polymerisation of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties, including toughness, high elasticity and a tendency to form amorphous structures rather than crystals.
The polymer obtained from only one type of monomer unit is called a homopolymer. The polymer obtained by the simple addition of more than one monomer unit or condensation(release of material during the reaction) of more than one type of monomer unit having bi or more functional groups is called a copolymer.
On the basis of used monomer polymer can be divided into two categories:
a) Homopolymer b) Copolymer
PVC (polyvinyl chloride) is a homopolymer.

Polyvinyl chloride is produced in an additional polymerisation reaction using the chloroethene (vinyl chloride) monomer. This polymerisation reaction proceeds by a free-radical mechanism.
Nylon 6,6 is a copolymer.
Nylon -6,6 is synthesized by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/carboxylate mixture. The nylon salt goes into a reaction vessel where the polymerization process takes place either in batches or continuously.

Note: Homopolymer can be formed simply an additional reaction but copolymer not.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




