
Give description of extraction of sulphur by Frasch process with labelled diagram.
Answer
584.7k+ views
Hint: Frasch process is a method to extract sulphur from the underground deposits. It is the only industrial method which is used in recovering the sulphur from elemental deposits.
Complete step by step answer:
Elemental sulphur is found in the deposits in the ground. There are two properties of sulphur that are used in its extraction process. These are its low melting point and low density. The extraction of sulphur is done by a process known as the Frasch process. In this process, superheated water is pumped into the sulphur deposit, The sulphur then melts and is then extracted by lifting it to the surface with compressed air. There are a few steps in this process for sulphur to be extracted.
First, a well is drilled into the soil for mineral deposits. Then after this, the superheated water is pumped into the deposit (this water was approximately heated to 170 degrees Celsius).
As a result of this, the sulphur in the deposit melts. It melts so easily because sulphur has a low melting point.
Remember that, although the sulphur is melted, it is still in the deposit and we need to extract it.
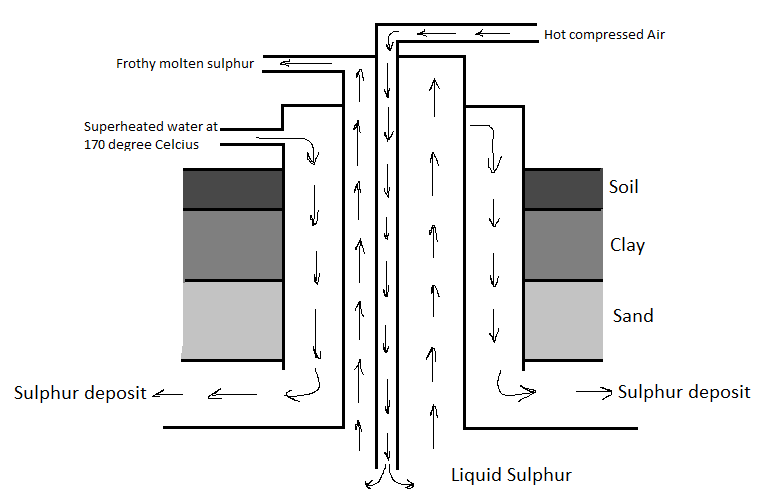
Now, besides with the water pumped into the well, compressed air is also pumped in it.
We know that sulphur has a low density and due to this reason, the molten sulphur comes to the surface.
The low density of sulphur allows the compressed air to push it to the surface.
Now, the sulphur can cool down and solidify since it is on the surface.
From here, sulphur forms a rhombic crystal.
Hence, in this way sulphur is extracted through the Frasch process.
Note: The Frasch process produces sulphur of highly pure quality. The recovered water from the Frasch process contains some dissolved minerals. The effluent needs to be cooled to avoid thermal pollution and should be recycled to avoid contamination of local ecosystems. Also once sulphur is extracted the caverns leftover are prone to subsidence (collapse). They are difficult to backfill and can become filled with groundwater over time.
Complete step by step answer:
Elemental sulphur is found in the deposits in the ground. There are two properties of sulphur that are used in its extraction process. These are its low melting point and low density. The extraction of sulphur is done by a process known as the Frasch process. In this process, superheated water is pumped into the sulphur deposit, The sulphur then melts and is then extracted by lifting it to the surface with compressed air. There are a few steps in this process for sulphur to be extracted.
First, a well is drilled into the soil for mineral deposits. Then after this, the superheated water is pumped into the deposit (this water was approximately heated to 170 degrees Celsius).
As a result of this, the sulphur in the deposit melts. It melts so easily because sulphur has a low melting point.
Remember that, although the sulphur is melted, it is still in the deposit and we need to extract it.

Now, besides with the water pumped into the well, compressed air is also pumped in it.
We know that sulphur has a low density and due to this reason, the molten sulphur comes to the surface.
The low density of sulphur allows the compressed air to push it to the surface.
Now, the sulphur can cool down and solidify since it is on the surface.
From here, sulphur forms a rhombic crystal.
Hence, in this way sulphur is extracted through the Frasch process.
Note: The Frasch process produces sulphur of highly pure quality. The recovered water from the Frasch process contains some dissolved minerals. The effluent needs to be cooled to avoid thermal pollution and should be recycled to avoid contamination of local ecosystems. Also once sulphur is extracted the caverns leftover are prone to subsidence (collapse). They are difficult to backfill and can become filled with groundwater over time.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




