
Give the major product of the following reaction:
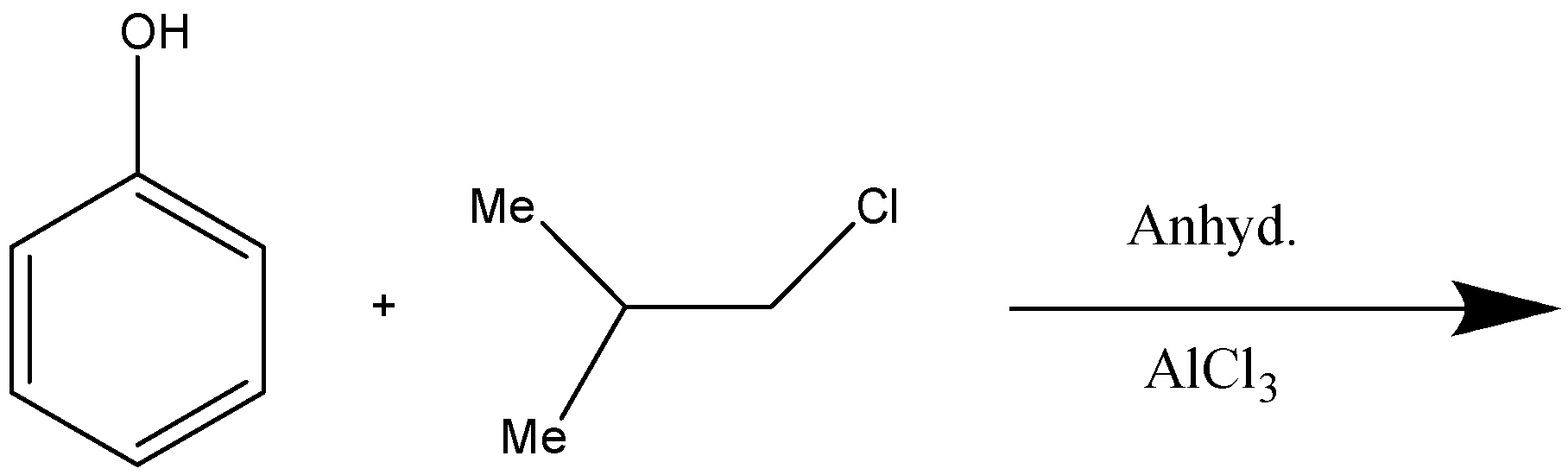

Answer
517.2k+ views
Hint: Before solving this reaction, we need to understand about the ortho, para or meta directing groups then only the product can be formed using anhydrous $AlC{l_3}$ as a catalyst. After that we will get the product by taking ortho, para or meta directing groups into the account.
Complete answer: Let’s study about the ortho-para or meta directing groups first. So the substituents due to which the incoming substituents go to the ortho or para position, those substituents are called ortho-para directing groups. For example- electron donating groups such as $ - N{H_2}$, alkyl groups, etc. And same is for meta directing groups, that is, the substituents due to which the incoming the substituents go to meta position, then they are called meta directing groups. For example: electron withdrawing groups such as $ - CN$, $ - N{O_2}$, etc.
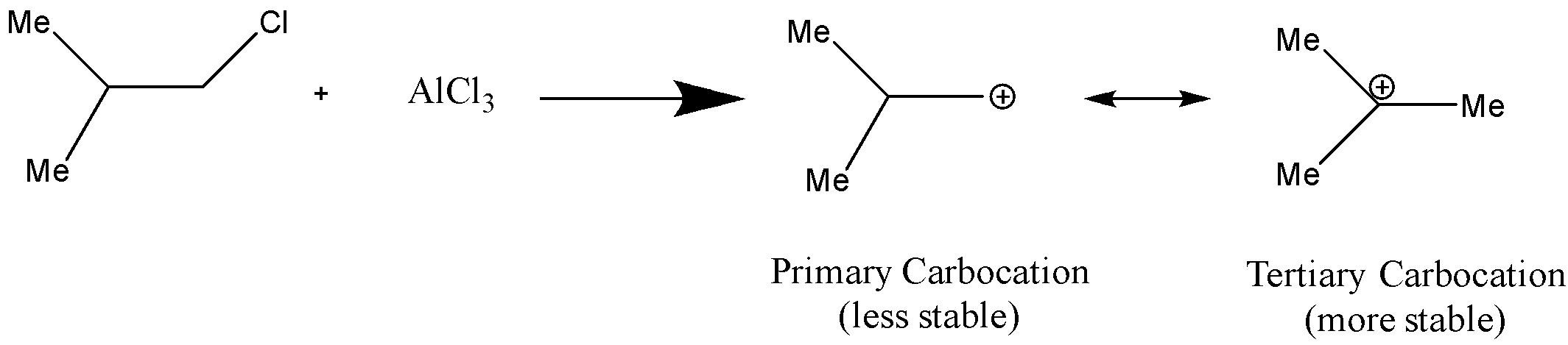
Here, this is a Friedel Craft Acylation reaction, it consists of acylation of the aromatic rings in the presence of a catalyst anhydrous $AlC{l_3}$. This is used as a catalyst because it works as a Lewis acid due to its ability to accept electrons by forming the intermediate products by the formation of the carbocation and speed up the reaction.
So, here we have phenol as a reactant, that is, benzene ring with $ - OH$group on it. Now, $ - OH$is an electron donating group so it is an ortho-para directing substituent, which means it will direct the incoming substituent to the ortho or para position. But here, in this case it will direct it to the para position because at the para position there is less steric hindrance as compared to the ortho position.
So, let’s see the product formed:
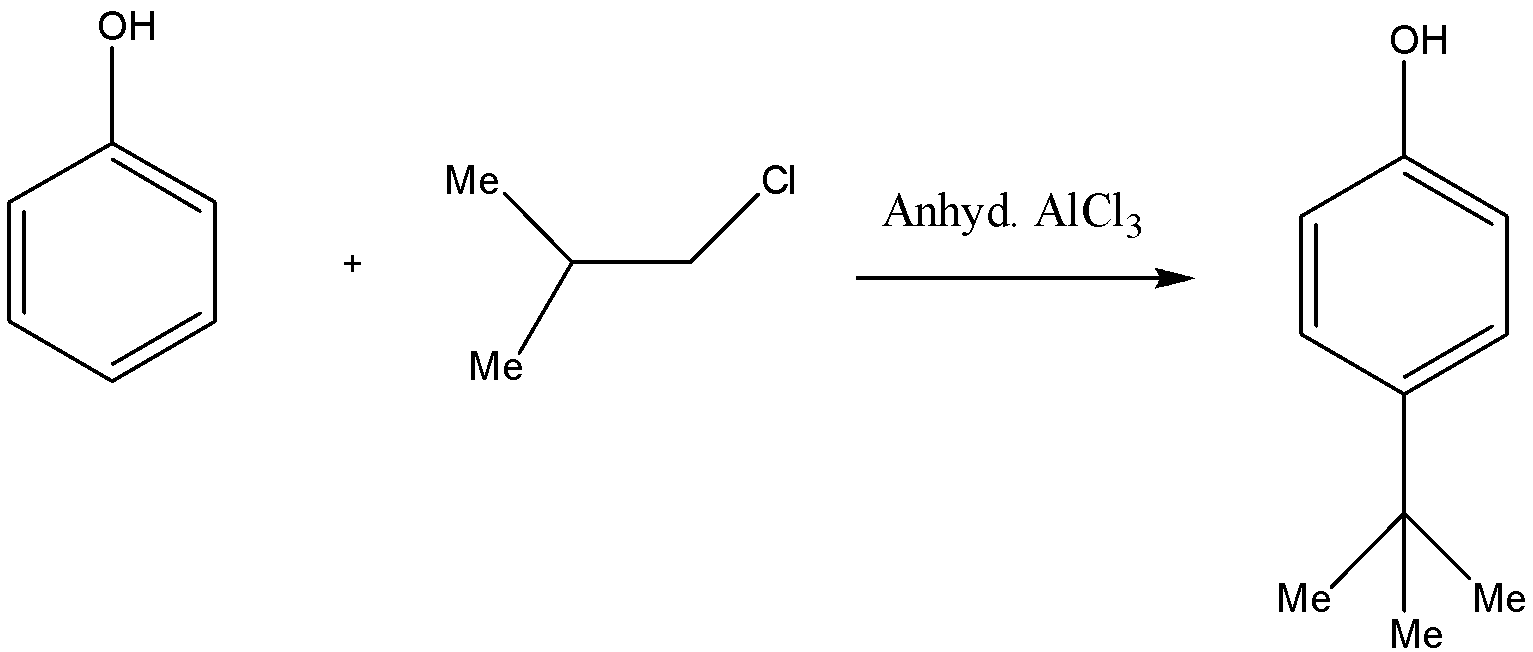
So, the product formed for this reaction will be:
Note:
In this question, the product was formed at para position instead of ortho position due to the steric hindrance. For such types of questions, we need to take care of the substituent present on the aromatic ring whether it is an ortho-para directing group or meta directing group and then accordingly the product will be formed.
Complete answer: Let’s study about the ortho-para or meta directing groups first. So the substituents due to which the incoming substituents go to the ortho or para position, those substituents are called ortho-para directing groups. For example- electron donating groups such as $ - N{H_2}$, alkyl groups, etc. And same is for meta directing groups, that is, the substituents due to which the incoming the substituents go to meta position, then they are called meta directing groups. For example: electron withdrawing groups such as $ - CN$, $ - N{O_2}$, etc.
Here, this is a Friedel Craft Acylation reaction, it consists of acylation of the aromatic rings in the presence of a catalyst anhydrous $AlC{l_3}$. This is used as a catalyst because it works as a Lewis acid due to its ability to accept electrons by forming the intermediate products by the formation of the carbocation and speed up the reaction.
So, here we have phenol as a reactant, that is, benzene ring with $ - OH$group on it. Now, $ - OH$is an electron donating group so it is an ortho-para directing substituent, which means it will direct the incoming substituent to the ortho or para position. But here, in this case it will direct it to the para position because at the para position there is less steric hindrance as compared to the ortho position.
So, let’s see the product formed:

So, the product formed for this reaction will be:

Note:
In this question, the product was formed at para position instead of ortho position due to the steric hindrance. For such types of questions, we need to take care of the substituent present on the aromatic ring whether it is an ortho-para directing group or meta directing group and then accordingly the product will be formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




